畜禽养殖废水和许多工业有机废水都具有高氨氮和低碳氮比的特点,城镇生活污水的碳氮比也呈现出下降趋势,给传统的生物脱氮处理技术带来了严峻挑战[1-3].在传统生物脱氮处理工艺基础上,增加铵(NH4+)的物化去除设施,或外加碳源以调升废水的碳氮比,是目前广为采用的以达到良好脱氮效果的措施,但也显著提高了废水处理成本[2, 4].厌氧氨氧化(anaerobic ammonium oxidation,ANAMMOX)以亚硝酸盐(NO2-)和NH4+分别作为电子受体和电子供体发生反应,生成N2和少量硝酸盐(NO3-),被认为是最为经济高效的生物脱氮途径,适宜于高氨氮低碳氮比废水的脱氮处理[5].然而,ANAMMOX菌群的增殖缓慢,在成分复杂的废水或污水生物处理系统中,其生长代谢极易受到增殖较快的化能异养微生物的抑制,如何实现有效富集并使之与其他功能菌群达到代谢上的平衡,是ANAMMOX技术工程应用的关键[6-9].
研究表明,在溶解氧(DO)为0.3~1.0 mg/L的微氧(亦称限氧)条件下,好氧、厌氧和兼性细菌可共存于同一污泥相中,在单一反应器中即可实现碳氮的同步去除,具有工程投资省、剩余污泥少、耗能低等优点[10-12].针对干清粪养猪废水高氨氮低碳氮比的特点,前期设计并启动运行了升流式限氧生物膜反应器(upflow oxygen limitation biofilm reactor,UOLBR),并成功富集了ANAMMOX功能菌群,在水力停留时间(HRT)8 h、27 ℃和DO为0.40 mg/L的条件下,对化学需氧量(COD)、铵态氮(NH4+-N)和总氮(TN)的去除负荷分别平均高达0.60,0.94和0.91 kg/(m3 · d),实现了碳氮的高效同步去除,但启动进程缓慢,达180 d以上[13].在实际应用中,污水和废水生物处理系统还会因季节性生产、年度维护及设备故障等原因而中断运行数天乃至数月.重新启动运行时,系统往往需要一定的恢复期以达到良好的处理效能[14-15].因此,实现生物处理系统闲置后的快速恢复对实际工程应用具有重要意义.本文在前期启动运行并实现以ANAMMOX为主导的脱氮途径后,将UOLBR闲置2个月,在HRT 10 h、25 ℃和出水回流比25: 1的条件下再次启动,考察其处理效果和ANAMMOX功能的恢复情况,并对系统的微生物群落结构和脱氮途径进行分析,以期为限氧生物处理系统的启动和运行管理提供指导.
1 实验 1.1 实验装置图 1为UOLBR养猪废水处理系统的装置示意.其中,UOLBR由有机玻璃制成,反应区域容积4.9 L,反应柱高0.5 m,内径0.1 m,顶部设有固-液-气三相分离器,底部为锥形,与进水管和出水回流管连接.在反应器内的中上部,装填有规格为Φ16×10 mm的PVC填料,填料床高200 mm,自然堆积孔隙率在95%左右.UOLBR进水由蠕动泵泵入,顶部出水排入容积为10 L蓄水箱.蓄水箱一分为二,其一用于缓存排放出水,另一部分用于存储出水并曝气,由蠕动泵回流以维持UOLBR内的限氧环境.UOLBR配有在线溶解氧监测仪对回流水的曝气量进行控制,将反应系统的DO维持在设定值.UOLBR外壁缠有电热丝,由温控仪将反应器内的温度控制在(25±1)℃.
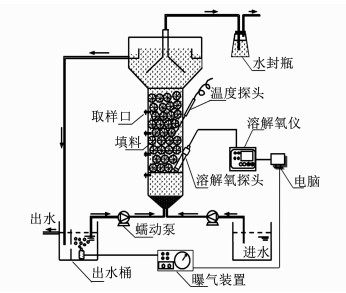
|
图 1 UOLBR系统装置示意 Fig. 1 Schematic diagram of the UOLBR system |
实验用水为取自哈尔滨市某养猪场的干清粪养猪废水,其COD、NH4+-N和TN质量浓度分别为217~1 410,104.3~471.6和104.9~472.5 mg/L,COD与TN比在0.7~4.3内波动,是典型的高氨氮低碳氮比有机废水.基于前期研究,利用序批式反应器(sequencing batch reactor,SBR)对养猪废水进行预处理,通过COD的去除,将UOLBR进水的COD与TN比控制在0.6~1.0内[13, 16-17].在重新启动运行过程中,UOLBR进水的COD、NH4+-N和TN质量浓度分别为114~263,171.4~281.7和171.6~281.1 mg/L.
1.3 UOLBR前期和重新启动的控制参数UOLBR在前期已成功启动并连续运行[13].在HRT 10 h、25 ℃、出水回流比25: 1,以及进水COD、NH4+-N、TN和pH分别为(193±35),(251.2±31.1),(251.9±31.0) mg/L和(8.2±0.2)的条件下,UOLBR在稳定运行状态下对COD、NH4+-N和TN的平均去除率分别为62.3%, 95.3%和88.3%,此时反应器内悬浮污泥的混合液悬浮固体(MLSS)和挥发性悬浮固体(MLVSS)总量分别为5.18和3.60 g/L,生物膜的分别为8.47和2.81 g/L.在满水状态下,反应器停止运行2个月,之后重新启动并连续运行,HRT、温度和出水回流比等控制参数与停运前相同.依据系统内的DO水平,UOLBR的重新启动运行过程分为两个阶段:前30 d为第1阶段,将DO控制在2.5~3.0 mg/L的较高水平,以富集硝化菌群;自第31天以后的运行为第2阶段,将DO调控为0.2~0.5 mg/L,使系统处于限氧状态并持续运行.UOLBR在这两个阶段的控制参数和水质如表 1所示.
| 表 1 UOLBR重新启动过程的阶段和控制参数 Tab. 1 Stages and control parameters within the restart-up process of the UOLBR |
在UOLBR的启动运行过程中,每天定时采集进水和出水样品进行水质分析.COD、NH4+、NO2-和NO3-分别采用重铬酸钾法、纳式试剂光度法、N-(1-萘基)-乙二胺光度法和紫外分光光度法[18].pH采用pH计(Switzerland Mettler Toledo,DELTA 320)检测,DO采用溶解氧在线检测仪(台湾衡欣,AZ8403)测定.水样中的TN以NH4+-N、NO2--N及NO3--N之和计.
1.5 生物量与微生物群落结构分析在UOLBR重新启动运行的第68天,从填料床随机采集填料3个,利用涡漩振荡器(Kylin-bell,VORTEX-5)将填料上的生物膜剥离,烘干;从反应器侧面最下端取样口(图 1)采集泥水混合液100 mL,烘干.将以上生物膜和悬浮污泥恒重,并计算系统内的MLSS和MLVSS[18].
借助于细菌16S rRNA基因的高通量测序,分别对悬浮污泥和生物膜污泥进行微生物群落结构分析.其中,样品的总DNA利用Power soil DNA试剂盒(美国,MOBIO)提取,细菌16S rRNA基因的V3~ V4区扩增引物为341F(5′-CCTACGGGAGGCAGCAG-3′)和805R(5′-GACTACHVGGGTATCTAATCC-3′),PCR产物的高通量测序仪器为Illumina Miseq PE300(美国,Illumina)[16].依据高通量测序提供的信息,进行微生物群落多样性和构成分析[19].
2 结果与讨论 2.1 COD的去除如图 2所示,UOLBR系统在重新启动运行的第1天,其COD去除率为59.2%.随着运行的延续,系统对COD的去除率逐渐上升,并在第1阶段的最后10 d(第19~第30天)维持在62.1%左右.自第31天进入第2运行阶段后,尽管系统中的DO由第1阶段的2.68 mg/L降低到0.42 mg/L左右,但UOLBR对COD的去除率并未受到显著影响.在运行的最后16 d(第53~第68天),UOLBR的COD去除率平均为64.0%,出水COD平均只有61 mg/L.经过68 d的恢复运行,UOLBR系统悬浮污泥的MLSS和MLVSS分别为6.38和4.09 g/L,生物膜的分别为9.27和2.95 g/L,表征污泥活性的MLVSS与MLSS比分别为0.64和0.32,与反应器在2个月前停运时的0.70和0.33相近.以上结果表明,尽管UOLBR闲置长达2个月之久,但其中的微生物仍然保持着良好的活性,对COD的去除能力可得到迅速恢复.
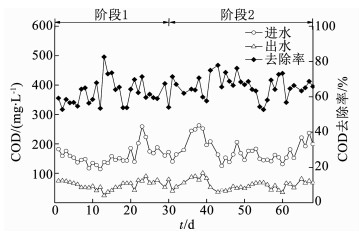
|
图 2 UOLBR在重新启动过程的COD去除 Fig. 2 COD removal in the UOLBR during the restart-up process |
干清粪养猪废水是典型的高氨氮低碳氮比有机废水,有效脱氮是其处理的核心和难点[13, 16-17].由于干清粪养猪废水的TN主要由NH4+-N贡献(表 1),欲使UOLBR达到良好的脱氮效能,首先必须使氨氧化菌群(AOB)得到富集,将NH4+-N氧化为NO2--N.为此,在UOLBR重新启动运行的第1阶段,将系统内的DO控制在了2.68 mg/L这一较高的水平(表 1).如图 3(a)所示,UOLBR在重新启动运行的第1天,由于反应器内混合液对进水的稀释作用,其NH4+-N去除率高达97.1%,但随着运行的延续,系统表现出NH4+-N去除率持续下降,至第1阶段在第30天结束时,只有57.9%.尽管NH4+-N去除率不断下降,出水中的NO3--N呈现出持续增加趋势(图 3(b)),在第30天达到了52.2 mg/L,表明包括AOB和亚硝酸盐氧化菌群(NOB)在内的硝化菌群得到了富集和活性恢复.由于较高的DO水平会严重抑制包括反硝化和ANAMMOX等脱氮功能菌群的活性[20],在DO为2.68 mg/L左右的第1运行阶段,UOLBR并未呈现出显著的脱氮效应,所呈现出的TN去除率逐步下降的趋势,主要也是由反应器内原有混合液对进水的稀释作用引起的(图 3 (c)).
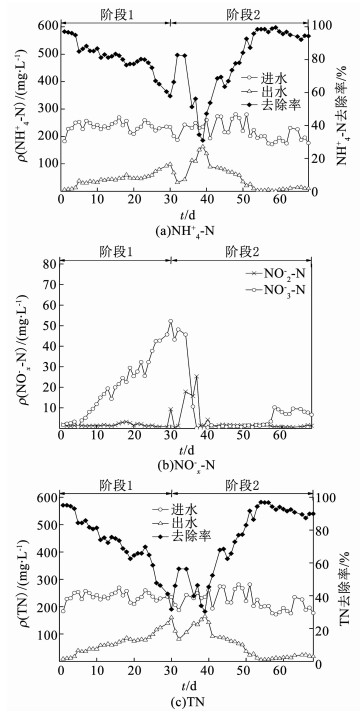
|
图 3 NH4+-N、NOx--N和TN在UOLBR重新启动过程中的变化 Fig. 3 NH4+-N, NOx--N, and TN in the UOLBR during the restart-up process |
自第31天将UOLBR内的DO下调到0.42 mg/L左右后(表 1),系统对NH4+-N的去除虽有明显波动,但仍表现为继续下降趋势,直到第39天达到最低值30.9%(图 3a).同一时期,TN去除率则表现出了恢复趋势,但在第39天也只有30.4%(图 3(c)).在第31~第39天的运行中,由于DO的显著降低,硝化作用受到了显著影响,出水中的NO3--N迅速下降,同时出现了NO2--N的短期积累(图 3(b)).第39天以后,UOLBR的NH4+-N和TN去除率均呈现出了快速增加趋势,并在最后的16 d(第53~第68天)保持了相对稳定,分别平均高达96.5%和91.7%,出水平均质量浓度分别只有7.0和16.4 mg/L,说明系统的生物脱氮功能得到了有效恢复.值得注意的是,在最后16 d的稳定运行期,UOLBR系统中出现了一定量的NO3--N积累,出水质量浓度平均为8.2 mg/L,而NO2--N质量浓度则保持在1.0 mg/L左右的低水平.这一结果暗示,ANAMMOX在系统生物脱氮功能中发挥了重要作用[5, 10].
2.3 UOLBR系统的微生物群落结构分析为了解UOLBR恢复后的脱氮机制,在最后的稳定运行期分别采集悬浮污泥和生物膜,借助于细菌16S rRNA基因的高通量测序技术,对其微生物群落结构进行了分析.
如表 2所示,从UOLBR悬浮污泥和生物膜样品中分别获得47 446和47 922条基因序列,以及1 725和1 916个操作分类单元(operational taxonomic units,OTUs),2个样品的基因文库覆盖率均达到了99%,说明测序结果能够反映样本的真实情况.Alpha多样性可以反映微生物群落的丰度和多样性,其中Chao1和ACE指数表征的是群落丰度,Shannon-Wiener和Simpson指数表征的是群落多样性[21].比较分析发现(表 2),在UOLBR系统中,悬浮污泥与生物膜微生物群落的物种丰富度都很高,但生物膜中的物种丰度和多样性均高于悬浮污泥,说明填料的布设更利于复杂微生物群落的构建,具有提升系统生物量和污染物降解途径的显著功效.
| 表 2 UOLBR悬浮污泥和生物膜的Alpha多样性分析 Tab. 2 Alpha diversity of the sampled suspended sludge and biofilm in the UOLBR |
基于分类到属的OTUs分析发现,功能恢复后的UOLBR系统,其悬浮污泥中存在大量与生物脱氮相关的功能菌群,如硝化菌群、反硝化菌群和ANAMMOX菌群等(图 4(a)).其中,硝化菌群相对丰度为0.41%(包括0.29%的AOB和0.12%的NOB),AOB主要有Nitrosomonas[22],Prosthecobacter[23]和Sphingomonas[24],NOB主要有Nitrobacter[25]和Nitrospira[26];反硝化菌群的相对丰度为14.87%,主要包括Limnobacter[27]、Azospira[28]、Methyloversatilis[29]、Azohydromonas[30]、Lautropia[31]和Longilinea[32],相对丰度分别为4.06%,2.66%,1.48%,1.13%,1.09%和1.05%;ANAMMOX菌群的相对丰度为0.78%,主要是Candidatus brocadia、C.kuenenia和C.anammoxoglobus[33],相对丰度分别为0.58%,0.20%和0.002%.
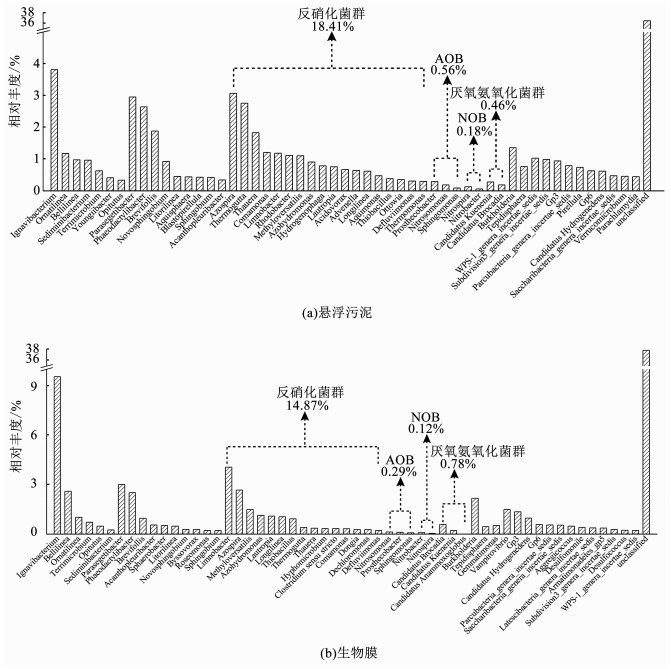
|
图 4 UOLBR悬浮污泥和生物膜的菌属相对丰度 Fig. 4 Relative abundance of genus in suspended sludge and biofilm of the UOLBR |
如图 4(b)所示,硝化菌群,包括Nitrosomonas、Prosthecobacter和Sphingomonas在内的AOB,以及包括Nitrobacter和Nitrospira在内的NOB,同样大量存在于UOLBR的生物膜中,但其总体相对丰度均比悬浮污泥中的高,分别达到了0.56%和0.18%.生物膜中的反硝化菌群结构与悬浮污泥的有显著差异,优势菌属主要是Azospira[28]、Thermogutta[34]、Thauera[28]、Comamonas[35]、Limnobacter[27]、Rhodobacter[28]和Methyloversatilis[29],其相对丰度分别为3.07%,2.76%,1.83%,1.21%,1.18%,1.11%和1.1%,总体丰度达到了18.41%,显著高于悬浮污泥的14.87%.与悬浮污泥相比,生物膜中的ANAMMOX菌属相对丰度较低,为0.46%,主要包括C.kuenenia(0.28%)和C.brocadia(0.18%).
比较发现[36],在停止运行的UOLBR中,其AOB、NOB、反硝化菌群和ANAMMOX菌群的相对丰度范围分别为0.25%~4.10%,0.10%~0.34%,11.43%~19.17%和0.08%~0.93%.而在重新启动并达到稳定运行状态后,UOLBR中的AOB、NOB、反硝化细菌和ANAMMOX的相对丰度均达到或超过了停止运行前的水平.
以上结果表明,悬浮污泥和生物膜的共存,不仅可以使AOB、NOB、硝酸盐还原菌群、亚硝酸盐还原菌群和ANAMMOX菌群等共栖于UOLBR限氧生物处理系统中,而且增加了生物脱氮功能菌群的多样性,为全程硝化反硝化脱氮、短程硝化反硝化脱氮和ANAMMOX脱氮奠定了生物学基础.
2.4 UOLBR系统的脱氮途径分析除了细胞合成外,废水生物脱氮途径有三,即全程硝化反硝化、短程硝化反硝化和ANAMMOX[19, 20].图 4所示的微生物群落结构表明,在UOLBR系统中,共栖着AOB、NOB、硝酸盐还原菌、亚硝酸盐还原菌和ANAMMOX菌等生物脱氮功能菌群,全程硝化反硝化、短程硝化反硝化和ANAMMOX均有发生的可能.如式(1)和式(2)所示,NO3--N和NO2--N的反硝化脱氮均需有机碳源作为电子供体,而ANAMMOX的脱氮过程(式(3))无需有机碳源[5, 37].
| $ 5\text{C+2}{{\text{H}}_{\text{2}}}\text{O+4NO}_{3}^{-}\to 2{{\text{N}}_{2}}+4\text{O}{{\text{H}}^{\text{-}}}+5\text{C}{{\text{O}}_{2}}, $ | (1) |
| $ 3\text{C+2}{{\text{H}}_{\text{2}}}\text{O+4NO}_{2}^{-}\to 2{{\text{N}}_{2}}+4\text{O}{{\text{H}}^{\text{-}}}+3\text{C}{{\text{O}}_{2}}, $ | (2) |
| $ \begin{align} &\text{NH}_{4}^{+}+1.32\text{NO}_{2}^{-}+0.066\text{HCO}_{3}^{-}+0.13{{\text{H}}^{+}}\to \\ &1.02{{\text{N}}_{2}}+0.26\text{NO}_{3}^{-}+0.066\text{C}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{0}\text{.15}}}+2.03{{\text{H}}_{2}}\text{O}\text{.} \\ \end{align} $ | (3) |
由式(1)、(2)可知,通过反硝化作用,将NO3--N还原为N2所需的COD去除与TN去除比为2.86,还原NO2--N所需要的COD去除与TN去除比也要达到1.71.如图 2和图 3(c)所示,UOLBR在第53~第68天的相对稳定运行阶段,其COD和TN去除率分别平均为64.0%和91.7%,COD去除与TN去除比平均为0.61,且没有明显的NO2--N积累(图 3(b)).假设系统去除的COD全部被用于NO3--N或NO2--N的还原,全程硝化反硝化和短程硝化反硝化对系统TN去除率的最大贡献分别也只有21.33%和35.67%.因此,在处于稳定运行时期的UOLBR系统中,至少有64.33%的TN是由ANAMMOX途径贡献的.可见,恢复后的UOLBR系统保持了生物脱氮途径的多样性,而ANAMMOX仍是其最主要的生物脱氮途径[13].
3 结论1) 闲置2个月之久的UOLBR,可直接重新启动,并在53 d内达到稳定运行.在HRT 10 h、25 ℃和出水回流比25: 1的条件下,其COD、NH4+-N和TN去除率分别维持在64.0%,96.5%和91.7%的较高水平.
2) 生物脱氮功能菌群在UOLBR悬浮污泥和生物膜中的分布存在显著差异.其中,生物膜富集了更多的硝化菌群和反硝化菌群,而悬浮污泥中的ANAMMOX更为丰富.
3) 功能恢复后的UOLBR系统,保留了全程硝化反硝化、短程硝化反硝化以及ANAMMOX等多种脱氮功能菌群及脱氮途径共存的特征,其中ANAMMOX依然是系统的主要脱氮途径.
| [1] |
OTHMAN I, ANUAR A N, UJANG Z, et al. Livestock wastewater treatment using aerobic granular sludge[J]. Bioresource Technology, 2013, 133: 630. DOI:10.1016/j.biortech.2013.01.149 |
| [2] |
KUMAR M, LEE P Y, FUKUSIHMA T, et al. Effect of supplementary carbon addition in the treatment of low C/N high-technology industrial wastewater by MBR[J]. Bioresource Technology, 2012, 113: 148. DOI:10.1016/j.biortech.2011.12.102 |
| [3] |
DU Rui, CAO Shenbin, WANG Shuying, et al. Performance of partial denitrification (PD)-ANAMMOX process in simultaneously treating nitrate and low C/N domestic wastewater at low temperature[J]. Bioresource Technology, 2016, 219: 420. DOI:10.1016/j.biortech.2016.07.101 |
| [4] |
HUANG Haiming, CHEN Yongqiang, JIANG Yang, et al. Treatment of swine wastewater combined with MgO-saponification wastewater by struvite precipitation technology[J]. Chemical Engineering Journal, 2014, 254: 418. DOI:10.1016/j.cej.2014.05.054 |
| [5] |
VAN D U, JETTEN M, VAN L M. The SHARON-Anammox process for treatment of ammonium rich wastewater[J]. Water Science and Technology: A Journal of the International Association on Water Pollution Research, 2001, 44(1): 153. DOI:10.2166/wst.2001.0037 |
| [6] |
STROUS M, HEIJNEN J J, KUENEN J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms[J]. Applied Microbiology and Biotechnology, 1998, 50(5): 589. DOI:10.1007/s002530051340 |
| [7] |
LING Jian, CHEN Shulin. Impact of organic carbon on nitrification performance of different biofilters[J]. Aquacultural Engineering, 2005, 33(2): 150. DOI:10.1016/j.aquaeng.2004.12.002 |
| [8] |
NI Shouqing, NI Jianyuan, HU Deliang, et al. Effect of organic matter on the performance of granular anammox process[J]. Bioresource Technology, 2012, 110: 701. DOI:10.1016/j.biortech.2012.01.066 |
| [9] |
CHU Zhaorui, WANG Ke, LI Xiangkun, et al. Microbial characterization of aggregates within a one-stage nitritation-anammox system using high-throughput amplicon sequencing[J]. Chemical Engineering Journal, 2015, 262: 41. DOI:10.1016/j.cej.2014.09.067 |
| [10] |
WANG Jianlong, YANG Ning. Partial nitrification under limited dissolved oxygen conditions[J]. Process Biochemistry, 2004, 39(10): 1223. DOI:10.1016/S0032-9592(3)00249-8 |
| [11] |
ZHENG Shaokui, LI Huijun, CUI Cancan. An upflow microaerobic sludge blanket reactor operating at high organic loading and low dissolved oxygen levels[J]. Biotechnology Letters, 2011, 33(4): 693. DOI:10.1007/s10529-010-0505-4 |
| [12] |
NIU Tianhao, ZHOU Zhen, SHEN Xuelian, et al. Effects of dissolved oxygen on performance and microbial community structure in a micro-aerobic hydrolysis sludge in situ reduction process[J]. Water Research, 2016, 90: 369. DOI:10.1016/j.watres.2015.12.050 |
| [13] |
王成, 孟佳, 李玖龄, 等. 升流式微氧生物膜反应器处理高氨氮低碳氮比养猪废水的效能[J]. 化工学报, 2016, 67(9): 3895. WANG Cheng, MENG Jia, LI Jiuling, et al. Pollutant removal efficiency in upflow microaerobic biofilm reactor treating manure-free piggery wastewater with low COD/TN ratio and high NH4+-N[J]. CIESC Journal, 2016, 67(9): 3895. |
| [14] |
ELAWWAD A, SANDNER H, KAPPELMEYER U, et al. Long term starvation and subsequent recovery of nitrifiers in aerated submerged fixed bed biofilm reactors[J]. Environmental Technology, 2013, 34(8): 945. DOI:10.1080/09593330.2012.722758 |
| [15] |
YILMAZ G, LEMAIRER R, KELLER J, et al. Effectiveness of an alternating aerobic, anoxic/anaerobic strategy for maintaining biomass activity of BNR sludge during long-term starvation[J]. Water Research, 2007, 41(12): 2590. DOI:10.1016/j.watres.2007.02.011 |
| [16] |
LI Jianzheng, MENG Jia, LI Jiuling, et al. The effect and biological mechanism of COD/TN ratio on nitrogen removal in a novel upflow microaerobic sludge reactor treating manure-free piggery wastewater[J]. Bioresource Technology, 2016, 209: 360. DOI:10.1016/j.biortech.2016.03.008 |
| [17] |
何佳敏, 孟佳, 张永, 等. 温度降低对UMSR处理高氨氮低碳氮比养猪废水效能的影响[J]. 化工学报, 2017, 68(5): 2074. HE Jiamin, MENG Jia, ZHANG Yong, et al. Effect of lower temperature on performance of upflow microaerobic sludge reactor treating manure-free piggery wastewater with high NH4+-N and low COD/TN ratio[J]. CIESC Journal, 2017, 68(5): 2074. |
| [18] |
国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2006. Ministry of Environmental Protection. Detection and analysis method of water and wastewater[M]. 4th ed. Beijing: China Environment Science Press, 2006. |
| [19] |
MENG Jia, LI Jiuling, LI Jianzheng, et al. Effect of reflux ratio on nitrogen removal in a novel upflow microaerobic sludge reactor treating piggery wastewater with high ammonium and low COD/TN ratio: Efficiency and quantitative molecular mechanism[J]. Bioresource Technology, 2017, 243: 922. DOI:10.1016/j.biortech.2017.07.052 |
| [20] |
MENG Jia, LI Jiuling, LI Jianzheng, et al. Nitrogen removal from low COD/TN ratio manure-free piggery wastewater within an upflow microaerobic sludge reactor[J]. Bioresource Technology, 2015, 198: 884. DOI:10.1016/j.biortech.2015.09.023 |
| [21] |
汪瑶琪, 张敏, 姜滢, 等. 厌氧氨氧化启动过程及微生物群落结构特征[J]. 环境科学, 2017, 38(12): 5184. WANG Yaoqi, ZHANG Min, JIANG Ying, et al. Start-up and characteristics of microbial community structure of ANAMMOX[J]. Environmental Science, 2017, 38(12): 5184. |
| [22] |
IGARASHI N, MORIYAMA H, FUJIWARA T, et al. The 2.8 angstrom structure of hydroxylamine oxidoreductase from a nitrifying chemoautotrophic bacterium, Nitrosomonas europaea[J]. Nature Structural and Molecular Biology, 1997, 4(4): 276. DOI:10.1038/nsb0497-276 |
| [23] |
GONZALEZ M A, RODRIGUEZ S A, GARCIA R M J, et al. Performance and bacterial community dynamics of a CANON bioreactor acclimated from high to low operational temperatures[J]. Chemical Engineering Journal, 2016, 287: 557. DOI:10.1016/j.cej.2015.11.081 |
| [24] |
ZHENG Xuesong, YANG Hong, LI Daotang. Change of microbial populations in a suspended-sludge reactor performing completely autotrophic N-removal[J]. World Journal of Microbiology and Biotechnology, 2005, 21(6/7): 843. DOI:10.1007/s11274-004-5956-0 |
| [25] |
BOON B, LAUDELOUT H. Kinetics of nitrite oxidation by Nitrobacter winogradskyi[J]. Biochemical Journal, 1962, 85(3): 440. DOI:10.1042/bj0850440 |
| [26] |
DAIMS H, NIELSEN P, NIELSEN J, et al. Novel Nitrospira-like bacteria as dominant nitrite-oxidizers in biofilmsfrom wastewater treatment plants:Diversity and in situ physiology[J]. Water Science and Technology, 2000, 41(4/5): 85. |
| [27] |
SONG B, KERKHOF L J, HAGGBIOM M M. Characterization of bacterial consortia capable of degrading 4-chlorobenzoate and 4-bromobenzoate under denitrifying conditions[J]. FEMS Microbiology Letters, 2002, 213(2): 183. DOI:10.1016/S0378-1097(2)00804-2 |
| [28] |
ROSSI F, MOTTA O, MATRELLA S, et al. Nitrate removal from wastewater through biological denitrification with OGA 24 in a batch reactor[J]. Water, 2015, 7(1): 51. DOI:10.3390/w7010051 |
| [29] |
LI Enchao, WANG Rongchang, JIN Xuewen, et al. Investigation into the nitrate removal efficiency and microbial communities in a sequencing batch reactor treating reverse osmosis concentrate produced by a coking wastewater treatment plant[J]. Environmental Technology, 2017, 1. DOI:10.1080/09593330.2017.1352036 |
| [30] |
TUAN M N, KIM J. Azohydromonas riparia sp. nov. and Azohydromonas ureilytica sp. nov. isolated from a riverside soil in South Korea[J]. Journal of Microbiology, 2017, 55(5): 330. DOI:10.1007/s12275-017-6519-z |
| [31] |
SAMPAIO D S, BARBOZA A J R, DE JESUS H E, et al. Distribution of anaerobic hydrocarbon-degrading bacteria in soils from King George Island, Maritime Antarctica[J]. Microbial Ecology, 2017, 74(4): 810. DOI:10.1007/s00248-017-0973-3 |
| [32] |
ZHANG Lili, ZHANG Chao, HU Chengzhi, et al. Sulfur-based mixotrophic denitrification corresponding to different electron donors and microbial profiling in anoxic fluidized-bed membrane bioreactors[J]. Water Research, 2015, 85: 422. DOI:10.1016/j.watres.2015.08.055 |
| [33] |
GUO Jianhua, PENG Yongzhen, FAN Lu, et al. Metagenomic analysis of anammox communities in three different microbial aggregates[J]. Environmental Microbiology, 2016, 18(9): 2979. DOI:10.1111/1462-2920.13132 |
| [34] |
SLOBODKINA G B, KOVALEVA O L, MIROSHNICHENKO M L, et al. Thermogutta terrifontis gen. nov., sp. nov. and Thermogutta hypogea sp. nov., thermophilic anaerobic representatives of the phylum Planctomycetes[J]. International Journal of Systematic and Evolutionary Microbiology, 2015, 65: 760. DOI:10.1099/ijs.0.000009 |
| [35] |
DONG Qian, LIU Yanchen, SHI Hanchang, et al. Effects of graphite nanoparticles on nitrification in an activated sludge system[J]. Chemosphere, 2017, 182: 231. DOI:10.1016/j.chemosphere.2017.04.144 |
| [36] |
MENG Jia, LI Jiuling, LI Jianzheng, et al. Enhanced nitrogen removal from piggery wastewater with high NH4+ and low COD/TN ratio in a novel upflow microaerobic biofilm reactor[J]. Bioresource Technology, 2018, 249: 935. DOI:10.1016/j.biortech.2017.10.108 |
| [37] |
邓凯文, 李建政, 赵博玮. WFSI处理低碳氮比养猪废水的效果及脱氮机制[J]. 中国环境科学, 2016, 36(1): 87. DENG Kaiwen, LI Jianzheng, ZHAO Bowei. Efficiency and denitrification mechanism in a wood-chip-framework soil infiltrator treating piggery wastewater with low C/N ratio[J]. China Environmental Science, 2016, 36(1): 87. DOI:10.3969/j.issn.1000-6923.2016.01.014 |
 2019, Vol. 51
2019, Vol. 51


