类金刚石薄膜是以sp3、sp2杂化键结合为主体的长程无序三维网状结构,具有一系列特殊的物理和化学性能,如硬度高、化学稳定、高耐磨性等,在材料、机械、医学、航空航天等领域有广泛应用前景[1-2].但是DLC薄膜结合力较差,内应力较高,在较高的温度下会发生sp3→sp2转变,甚至石墨化[3-5].
氮元素掺杂既可以取代非晶碳基网络结构中的碳原子,也可以取代氢原子与碳原子键合[6],提高薄膜的热稳定性.同时碳氮键合引起薄膜结构弛豫从而使得残余内应力下降,可增强薄膜附着力[7-8].并且sp3杂化碳原子与氮原子键合(sp3C-N)可以形成类β-C3N4结构[9-10],可以缓解掺氮引起的sp2杂化含量增加而造成的硬度下降.有少量报道指出掺杂较低含量的氮有助于促进薄膜中碳-碳键以sp3形式存在[11].另外氮掺杂对DLC薄膜导电性具有明显的影响[12].
虽然已有上述研究报道,但宽氮含量范围内,氮对DLC薄膜组织结构的影响规律和机理认识尚有不足.本文控制氮气流量占总工作气体流量百分比在0~20%范围内,采用等离子增强化学气相沉积的方法制备a-C:H(N)薄膜,结合多种分析手段研究DLC薄膜在低氮掺杂时的碳氮键合形式、sp3杂化含量和力学性能的变化,并探讨其对薄膜组织结构影响机理,开发具有较高硬度和较低内应力的薄膜.这对a-C:H(N)薄膜的应用具有重要意义.
1 实验概况 1.1 a-C:H(N)薄膜的制备采用型号为Hauzer Flexicoat®850涂层设备,在316奥氏体不锈钢(20 mm×20 mm×5 mm)和Si(100)片(50 mm×10 mm×0.6 mm)上制备了WC/a-C:H(N)薄膜.先利用WC靶(99.99%),采用非平衡磁控溅射的方法制备过渡层;然后以乙炔(C2H2,99.999%)作为碳源,氮气作为氮源(N2,99.999%), 设置不同的N2/(C2H2+N2)流量比,采用PECVD的方法制备a-C:H(N)薄膜.具体工艺参数如表 1所示.本研究中镀膜前样品先后在丙酮、乙醇和去离子水中超声波辅助清洗20 min,以获得清洁表面.
| 表 1 a-C:H(N)薄膜沉积工艺参数 Tab. 1 Process parameters of a-C:H(N) thin film deposition |
为分析薄膜组织结构.采用岛津D/MAX-2500/PC型X射线衍射仪检测分析N-DLC薄膜结晶状态,薄膜化学键组成和相对百分比含量采用LabRAM Aramis拉曼光谱仪和日本Axis Ultra DLD型X射线光电子能谱仪进行分析.利用NOVA NANOSEM 430型扫描电镜对薄膜的表面形貌进行表征.
为了分析薄膜的性能.采用FST1000型薄膜应力测量仪检测硅片沉积前后的曲率,并利用如下所示的Stoney公式计算薄膜内应力[13].
| $ \sigma = \frac{{{E_{\rm{s}}}}}{{6(1 - {\upsilon _{\rm{s}}})}}\frac{{h_{\rm{s}}^2}}{{{h_{\rm{f}}}}}\left( {\frac{1}{R} - \frac{1}{{{R_{\rm{o}}}}}} \right). $ | (1) |
式中:Es为硅片的杨氏模量;hs为硅片的厚度;νs为硅片的泊松比;hf为薄膜的厚度;R0为镀膜前硅片的曲率半径;R为镀膜后的曲率半径.
采用纳米综合力学测量系统测量不同掺氮量薄膜的纳米硬度,加载力为5 mN,加载速率为10 mN/min,每个样品随机采集6个点,取其算术平均值.
2 结果及分析 2.1 氮掺杂对a-C:H(N)薄膜组织结构的影响针对不同N2/(C2H2+N2)流量比所制备的a-C:H(N)薄膜,利用XPS检测其中C1s和N1s的峰高比可知薄膜含氮量随着流量比变化趋势, 其结果如表 2所示.可知随着N2/(C2H2+N2)流量比增加,薄膜含氮量增加.即随着氮气流量增加,薄膜含氮量增加.图 1为不同掺氮量a-C:H(N)薄膜的XRD图谱,图中曲线均呈现出典型的非晶材料的“漫散射峰”,因此a-C:H(N)薄膜均为非晶碳膜.
| 表 2 不同N2/(C2H2+N2)流量比所制备a-C:H(N)薄膜含氮量变化 Tab. 2 Changes of nitrogen content in a-C:H(N) films prepared with different N2/(C2H2+N2) flow ratios |

|
图 1 a-C:H和N2.95at%-DLC薄膜XRD检测分析结果 Fig. 1 Detection and analysis of a-C:H and N2.95at%-DLC films by XRD |
图 2为不同N2/(C2H2+N2)流量比所制备的a-C:H(N)薄膜表面10 000倍SEM形貌图像.薄膜表面由尺寸和形状不一的非晶岛紧密排列组成.随着氮含量增加,岛状颗粒尺寸大小和不均匀程度增加.这可能与氮离子相对于氢的刻蚀作用较弱有关[14].本研究所采用偏压较高,随着掺氮量增加,氮离子数量增加,高能离子对薄膜表面刻蚀作用减弱,非晶岛状颗粒尺寸大小和表面粗糙程度增加.

|
图 2 不同N2/(C2H2+N2)流量比所制备a-C:H(N)薄膜表面形貌 Fig. 2 SEM morphologies of a-C:H(N) films prepared by different N2/ (C2H2+N2) flow ratios |
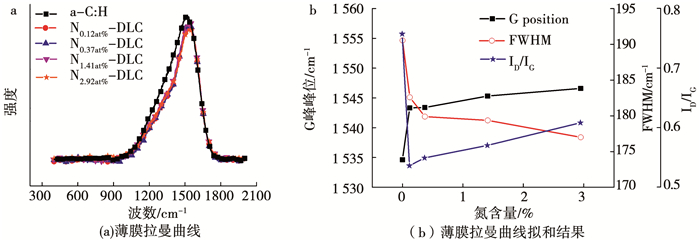
类金刚石薄膜的拉曼光谱可以利用高斯分峰拟合为波数在1 360 cm-1左右的D峰和波数1 580cm-1左右的G峰,其中D峰对应于环状sp2杂化键的振动,而G峰对应于环状或者链状sp2杂化键的振动[15, 16].a-C:H(N)薄膜拉曼曲线如图 3(a)所示,对拉曼曲线进行分峰拟合,结果如图 3(b)所示.其结果表明,随着氮掺杂含量的增加,存在一个氮掺杂量的临界值0.12 at%,ID/IG的比值先迅速下降后缓慢增加,G峰的半峰宽从迅速减小转变为缓慢减小,G峰的峰位向高波数方向从迅速移动转变为缓慢移动.而ID/IG的比值可以估算sp2团簇尺寸大小和sp3杂化的相对含量[17],G峰的半峰宽对键角和键长引起的结构紊乱敏感,G峰的峰位与sp2团簇的数量和大小有关[18-20].这表明随着氮的掺杂和含量的增加,sp2团簇的尺寸大小先减小后增加,非晶薄膜结构的紊乱程度下降,薄膜中sp3杂化含量先增加后减小.
|
图 3 a-C:H(N)薄膜拉曼曲线(a)和拟合分析结果(b) Fig. 3 Raman curves of a-C:H(N) films and fitting analysis results |
进一步分析薄膜各元素键合形式及其相对百分比,对a-C:H(N)薄膜进行XPS检测分析.在以往的文献中,C1s和N1s能级谱通过曲线拟合被分解成若干组分,而不同掺氮量下C1s不同组分的键能存在位移[12, 21-23].本研究将XPS检测得到的C1s和N1s能级谱进行校准和G-L拟合[24].N2.95at%-DLC薄膜C1s光谱分峰拟合为6个峰; sp2C=C(284.15eV), sp3C-C(284.83eV), C=N(285.7eV), C-O(286.3eV), C-N(287.15eV), C=O(288.17eV),其结果如图 4(a)所示.将N1s进行分峰拟合成4个峰:C-N(398.44eV), C≡N(399.22eV), C=N(399.49eV)和N-O(402eV),结果如图 4(b)所示.而a-C:H(N)薄膜中杂化键sp2C=C/sp3C-C比值和C=N/CN(C-N+C=N+C≡N)比值变化如图 5所示.图中氧峰可能来自于样品表面吸附氧和真空室氧气残留物[25].本文实验发现随着薄膜中氮含量自0到0.12 at%,sp2C=C/sp3C-C的比值迅速从0.65下降到0.563,当氮含量超出此上述范围,随着氮含量的增加,sp2C=C/sp3C-C的比值逐渐增加[11].以上变化趋势得到拉曼分析结果证实.显然氮掺杂影响了薄膜中sp3杂化含量,且存在一个临界氮掺杂值:当低于此值时,氮掺杂迅速地增加薄膜中的sp3杂化含量;而超过此值时则使薄膜中sp3杂化含量逐渐减少,即促进sp2团簇的形成.考虑氮替代薄膜中的元素而导致结构转变,不可能在零氮含量时发生结构突变,因此必然存在一个临界值.本文限于控氮手段限制,测试结果虽不尽精确,但0.12at%氮含量的临界值应算合理.

|
图 4 N2.95at%-DLC薄膜C1s能谱和N1s能谱分峰结果 Fig. 4 Peak splitting results of N2.95at%-DLC films by C1s and N1s spectra |

|
图 5 a-C:H(N)薄膜sp2C=C/sp3C-C比值和C=N/CN比值变化 Fig. 5 Changes of sp2C=C/sp3C-C and C=N/CN ratio of a-C:H(N) films |
从图 5也可以看出CN键的主要键合形式为C=N键,且随着氮掺杂含量的增加,C=N/CN比值下降[18].其原因可能是:CN键的键能比CC键键能高,而团簇界面处能量高,C=N键倾向于在界面处形成且与sp2C=C键构成sp2团簇,而C≡N键和C-N键在团簇界面处形成且与sp3C-C键构成sp3杂化[23, 26].本研究采用较高的偏压和控制线圈电流来提高工作气体的离化率.氮气流量低时,氮离子的能流密度低,而C=N键的键能较C-N键弱,氮离子倾向于与团簇界面处的不稳定碳形成C=N键[26].从而使薄膜中氮的键合形式主要为C=N键;随着氮气流量的增加,活性氮离子浓度增加,其能流密度增加,提高形成具有更高键能的键合形式的几率,C=N/CN比值下降.从氮元素键合形式的变化,可以探讨低氮掺杂对a-C:H(N)薄膜组织结构影响的可能机理:氮元素对薄膜sp3杂化含量的影响存在一个临界值.薄膜氮含量自0升到0.12 at%时,C=N键在团簇界面处的形成对sp2团簇起到钉扎作用,抑制sp2团簇形成长大,薄膜sp3杂化含量增加;当薄膜氮含量大于0.12at%时,C=N/CN比值下降,同理sp3杂化长大所受到的抑制作用增强而sp2团簇长大抑制作用减弱,促进sp2团簇尺寸大小和数量的增加.即氮元素的键合形式对薄膜sp3杂化和sp2团簇的形成和长大存在一个此消彼长的抑制作用,且其存在一个临界值.从中可知a-C:H(N)薄膜含氮量不同,氮的各种键合形式的百分占比发生变化,进而会影响薄膜的组织结构.
2.2 氮掺杂对a-C:H(N)薄膜力学性能的影响图 6为a-C:H(N)薄膜残余应力和纳米硬度检测结果.图 6(a)可以看出薄膜含氮量为0.12 at%时,薄膜残余应力为1.31 GPa,与a-C:H薄膜相比,残余应力迅速地下降了2.04 GPa.薄膜氮含量增加,薄膜残余应力虽有起伏,但总体呈缓慢下降趋势.a-C:H(N)薄膜残余应力变化可以用原位应力释放和钉扎效应来解释[27]:随着氮掺杂,氮与碳键合,而CN键的键长较CC键短,薄膜无序度下降,结构发生弛豫[28],残余应力大幅下降.而随着薄膜氮含量增加,大量的CN键在团簇界面处形成,形成钉扎效应,抑制薄膜结构的进一步弛豫,从而抑制了残余应力的下降.同时从图 6(b)中可知a-C:H(N)薄膜纳米硬度随着氮掺杂的进行呈下降趋势.在氮掺杂量低于0.12 at%时,薄膜中sp3含量虽较高,但是由于CN键的形成,结构发生弛豫,因而其硬度变化不大.但随着掺氮量的增加,sp3杂化含量下降,薄膜纳米硬度较快速地下降.

|
图 6 a-C:H(N)薄膜sp2C=C/sp3C-C比值和残余应力、纳米硬度变化 Fig. 6 Changes of sp2C=C/sp3C-C ratio with residual stress and nano-hardness of a-C:H(N) films |
1) 本文制备所得a-C:H(N)薄膜均为非晶碳膜.随着掺氮量增加,其ID/IG比值先迅速下降后缓慢增加,G峰的半峰宽从迅速减小转化为缓慢减小,G峰的峰位向高波数方向从迅速移动转变为缓慢移动,sp3杂化含量先增加后减少,非晶薄膜结构的紊乱程度下降.趋势变化出现拐点的薄膜氮含量为0.12 at%.
2) 本研究中氮与碳键合且主要以C=N键的形式存在.而氮元素的键合形式对a-C:H(N)薄膜sp3和sp2团簇的形成和长大存在一个此消彼长的抑制作用,且其存在一个含氮量临界值0.12 at%.掺氮量低时,C=N键百分占比高而抑制sp2团簇长大,使薄膜相比a-C:H薄膜有较高的sp3含量;随着薄膜氮含量增加,C=N/CN比值下降,对sp2团簇长大的抑制作用减弱,薄膜中sp3杂化含量下降.
3) 随着氮掺杂进行,氮与碳键合,键角无序度下降,薄膜结构发生弛豫,残余应力下降了2.04 GPa.而随着薄膜含氮量的进一步增加,薄膜残余应力变化不大.同时a-C:H(N)薄膜纳米硬度随薄膜含氮量增加而下降.当掺氮量较低时,可以获得表面平整均匀,具有较高硬度以及较低残余应力的薄膜.
| [1] |
Das D, BANERJEE A. Anti-reflection coatings for silicon solar cells from hydrogenated diamond like carbon[J]. Applied Surface Science, 2015, 345: 204. DOI:10.1016/j.apsusc.2015.03.124 |
| [2] |
UCUN I, ASLANTAS K, BEDIR F. The performance of DLC-coated and uncoated ultra-fine carbide tools in micromilling of Inconel 718[J]. Precision Engineering, 2015, 41: 135. DOI:10.1016/j.precisioneng.2015.01.002 |
| [3] |
CHIU H C, PENG L Y, WANG H Y, et al. High thermal stability of GaN Schottky diode with diamond-like carbon(DLC) anode design[J]. Journal of the Electrochemical Society, 2016, 163(3): H155-H158. DOI:10.1149/2.0241603jes |
| [4] |
JONGWANNASIRI C, LI X, WATANABE S. Improvement of thermal stability and tribological performance of diamond-like carbon composite thin films[J]. Materials Sciences and Applications, 2013, 4(10): 630. DOI:10.4236/msa.2013.410077 |
| [5] |
吴忠振, 田修波, 程思达, 等. 高结晶度CrN纳米粒子掺杂的DLC薄膜的显微结构及力学性能[J]. 金属学报, 2012, 48(3): 283. WU Z Z, TIAN X B, CHENG S D, et al. Microstructure and mechanical properties of DLC films doped with high crystallinity CrN nanoparticles[J]. Acta Metallurgica Sinica, 2012, 48(3): 283. DOI:10.3724/SP.J.1037.2011.00512 |
| [6] |
薛群基, 王立平. 类金刚石碳基薄膜材料[M]. 北京: 科学出版社, 2012: 112. XUE Q J, WANG L P. Diamond-like carbon film materials[M]. Beijing: Science Press, 2012: 112. |
| [7] |
LIN J F, WAN Z C, WEI P J, et al. Effect of nitrogen content on mechanical properties and tribological behaviors of hydrogenated amorphous carbon films prepared by ion beam assisted chemical vapor deposition[J]. Thin Solid Films, 2004, 466(1): 137. |
| [8] |
JAN D J, AI C F, LEE C C. Deposition of nitrogen-containing diamond-like carbon films on acrylic substrates by an ion beam process[J]. Vacuum, 2004, 74(3-4): 531. DOI:10.1016/j.vacuum.2004.01.024 |
| [9] |
WEI B, ZHANG B, JOHNSON K E. Nitrogen-induced modifications in microstructure and wear durability of ultrathin amorphous-carbon films[J]. Journal of Applied Physics, 1998, 83(5): 2491. DOI:10.1063/1.367009 |
| [10] |
ZOU Y S, WANG Q M, DU H, et al. Structural characterization of nitrogen doped diamond-like carbon films deposited by arc ion plating[J]. Applied Surface Science, 2005, 241(3-4): 295. DOI:10.1016/j.apsusc.2004.07.043 |
| [11] |
SUN L, LI H K, LIN G Q, et al. Influence of deposition parameters on the microstructure and properties of nitrogen-doped diamond like carbon films[J]. Journal of Vacuum Science & Technology A, 2010, 28(6): 1299. DOI:10.1116/1.3482010 |
| [12] |
TSUCHIYA M, MURAKAMI K, MAGARA K, et al. Structural and electrical properties and current-voltage characteristics of nitrogen-doped diamond-like carbon films on Si substrates by plasma-enhanced chemical vapor deposition[J]. Journal of Applied Physics, 2016, 55(6): 065502. DOI:10.7567/JJAP.55.065502 |
| [13] |
SHIRI S, ASHTIJOO P, ODESHI A, et al. Evaluation of Stoney equation for determining the internal stress of DLC thin films using an optical profiler[J]. Surface & Coatings Technology, 2016, 308. DOI:10.1016/j.surfcoat.2016.07.098 |
| [14] |
SETSUO N, TETSUO S, TSUTOMU S, et al. Optical and electrical properties of nitrogen-doped diamond-like carbon films prepared by a bipolar-type plasma-based ion implantation[J]. Japanese Journal of Applied Physics, 2012, 51(1). DOI:10.1143/JJAP.51.01AC04 |
| [15] |
HADDOCK D, PARKER T, SPINDLOE C, et al. Characterisation of diamond-like carbon (DLC) laser targets by Raman spectroscopy[C]. Journal of Physics: Conference Series, IOP Publishing, 2016: 012007. DOI: 10.1088/1742-6596/713/1/012007
|
| [16] |
FERRARI A C, ROBERTSON J. Interpretation of Raman spectra of disordered and amorphous carbon[J]. Physical Review B, 2000, 61(20): 14095. DOI:10.1103/PhysRevB.61.14095 |
| [17] |
TAMOR M A, VASSELL W C. Raman "fingerprinting" of amorphous carbon films[J]. Journal of Applied Physics, 1994, 76(6): 3823. DOI:10.1063/1.357385 |
| [18] |
CASIRAGHI C, FERRARI A C, ROBERTSON J. Raman spectroscopy of hydrogenated amorphous carbons[J]. Physical Review B Condensed Matter, 2005, 72(8): 85401. DOI:10.1103/PhysRevB.72.085401 |
| [19] |
BHATTACHARYYA S, CARDINAUD C, TURBAN G. Spectroscopic determination of the structure of amorphous nitrogenated carbon films[J]. Journal of Applied Physics, 1998, 83(8): 4491. DOI:10.1063/1.367211 |
| [20] |
杨义勇, 彭志坚, 付志强, 等. 多组分缓冲层W梯度掺杂DLC复合薄膜研究[J]. 金属学报, 2010, 46: 34. YaNG Y Y, PENG Z J, FU Z Q, et al. Study on W graded doping DLC composite films with multicomponent transition layer[J]. Acta Metallurgica Sinica, 2010, 46(46): 34. |
| [21] |
MANSOUR A, UGOLINI D. Photoelectron-spectroscopy study of amorphous a-CNx:H[J]. Physical Review B Condensed Matter, 1993, 47(16): 10201. DOI:10.1103/PhysRevB.47.10201· |
| [22] |
SCHARF T W, OTT R D, YANG D, et al. Structural and tribological characterization of protective amorphous diamond-like carbon and amorphous CNx overcoats for next generation hard disks[J]. Journal of Applied Physics, 1999, 85(6): 3142. DOI:10.1063/1.369654 |
| [23] |
SEKER Z, OZDAMAR H, ESEN M, et al. The effect of nitrogen incorporation in DLC films deposited by ECR Microwave Plasma CVD[J]. Applied Surface Science, 2014, 314(10): 46. DOI:10.1016/j.apsusc.2014.06.137 |
| [24] |
SHENG R D, LI L J, SU D Y, et al. Effect of unbonded hydrogen on amorphous carbon film deposited by PECVD with annealing treatment[J]. Diamond & Related Materials, 2018, 81: 146. DOI:10.1016/j.diamond.2017.12.002 |
| [25] |
HÖGSTRÖM J, ANDERSSON M, JANSSON U, et al. On the evaluation of corrosion resistances of amorphous chromium-carbon thin-films[J]. Electrochimica Acta, 2014, 122: 224. DOI:10.1016/j.electacta.2013.11.130 |
| [26] |
BHATTACHARYYA S, HONG J, TURBAN G. Determination of the structure of amorphous nitrogenated carbon films by combined Raman and x-ray photoemission spectroscopy[J]. Journal of Applied Physics, 1998, 83(7): 3917. DOI:10.1063/1.367312 |
| [27] |
马一博, 陈牧, 颜悦, 等. 薄膜应力测量方法及影响因素研究进展[J]. 航空材料学报, 2018, 38(1): 17. MA Y B, CHEN M, YAN Y, et al. Research progress of thin film stress measurement methods and influencing factors[J]. Journal of Aeronautical Materials, 2018, 38(1): 17. DOI:10.11868/j.issn.1005-5053.2017.000126 |
| [28] |
KHUN N W, LIU E, GUO H W. Cyclic voltammetric behavior of nitrogen-doped tetrahedral amorphous carbon films deposited by filtered cathodic vacuum arc[J]. Electroanalysis, 2010, 20(17): 1851. DOI:10.1002/elan.200804249 |
 2019, Vol. 51
2019, Vol. 51


