2. 环境生物学与污染控制教育部重点实验室(湖南大学),长沙 410082
2. Key Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, Changsha 410082, China
随着经济的快速发展,工业和农业活动频繁,大量有机化学物质积聚在土壤中[1-5].这些污染物通常具有“三致”效应,可能对人类健康和生态环境产生危害,因此,对有机污染土壤的治理成为当前面临的重要环境问题[6-13].微生物降解是有机污染物去除的重要途径[14-15].然而,生物降解速率通常受污染物低生物可利用性的限制[16].因此,探究土壤修复过程中有机污染生物可利用性的变化至关重要.
土壤改良剂,例如生物炭、堆肥腐殖质、表面活性剂等,既能改善土壤质地,提高土壤肥力,调节土壤酸碱度,又可促进污染物吸附或降解,在土壤修复中得到了广泛的应用[17-24].虽然已有研究证明,施用改良剂会改变土壤中有机污染物生物可利用性,但其作用机理尚不明确,没有统一的结论.本文总结了3种常用土壤改良剂对有机污染物生物可利用性的影响机理,包括生物炭、堆肥产物和表面活性剂,并为探究高效土壤改良剂提供一定参考方向.
1 生物可利用性2003年,美国国家研究委员会报告中将生物可利用性过程定义为影响生物暴露在含化学物质土壤或沉淀中发生的物理、化学和生物作用[25].目前,关于生物可利用性的定义尚不明确,统一认可的说法是污染物在土壤中的生物可利用性涉及到吸附和解吸,污染物运输和微生物的摄取这几个过程[26].因此,影响污染物水相浓度和微生物特征的因素将影响污染物生物可利用性[27].
土壤对有机污染物的吸附通常限制了其生物可利用性[28].随着时间的增加,土壤与有机污染物形成更强的结合力,导致其生物可利用性下降,该过程被称为老化.老化过程导致污染物解吸呈现3种形式:快速、缓慢和非常缓慢.可快速解吸的污染物通常是表面吸附在土壤颗粒上且可和孔隙水互换,因此,容易被微生物利用.而对于解吸缓慢和非常缓慢的部分,其被土壤强力吸附或进入到土壤微孔中,不易被微生物利用[29].有研究表明,某些微生物可以利用吸附态污染物,但对大多数微生物来说溶解态污染物更容易被利用[30].因此,有机污染物在土壤中的吸附和解吸是影响其生物有效性的关键过程.此外,当污染物处于低生物可利用性条件时,例如污染物溶解度较低,污染物与土壤强结合,吸附在土壤纳米孔径内部等,微生物特征显得尤为重要[15].通过污染物或微生物主动或被动地迁移,吸附态污染物生物可利用性得到提高.土壤改良剂通过作用于污染物在土壤中吸附和解吸,污染物和微生物之间传质以及土壤微生物丰度和活性,影响有机污染物生物可利用性.
2 土壤改良剂作用下有机污染物生物可利用性 2.1 生物炭生物炭是生物质材料在无氧或厌氧条件下热解产生的高度芳香化富含碳素的固态颗粒物.生物炭由于其稳定性高,原料来源广泛,价格低廉,且具有较强的固碳能力,常被用作土壤改良剂使农作物增产[18, 31-33].近年来,生物炭对污染物的吸附能力引起广泛关注,常用于污染土壤修复[34-35].
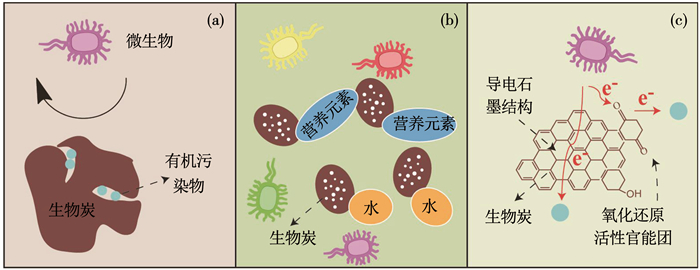
生物炭具有丰富的孔道结构、巨大的比表面积,以及丰富的表面官能团,对大部分污染物都有较强的吸附能力[36].据报道[37],土壤中加入1%生物炭对敌草隆的吸附能力是未添加生物炭土壤的7~80倍.生物炭的吸附作用可降低有机污染物的生态毒性,达到固定污染物的目的,同时,会导致水相溶液中有机污染物浓度的降低,抑制微生物对有机污染物的降解(如图 1(a)所示).Marchal等[38]通过实验观察和建模结果证明吸附动力学是限制菲生物降解的主要因素.Zhu等[39]也报道在沉淀中加入5%碳质材料后四溴联苯醚的微生物脱溴效率降低了92.8%~98.2%.
|
图 1 生物炭影响有机污染物生物可利用性机理 Fig. 1 Effect of biochar on the bioavailability of organic pollutants |
有研究发现,生物炭吸附作用并不总是抑制有机污染物的生物降解.某些微生物可通过直接附着或形成生物膜的方式,直接利用吸附态污染物.Xia等[40]利用扫描电镜发现黑炭孔径中存在Agrobacterium细菌.Rhodes等[41]也发现生物炭吸附态菲比用化学提取法得到的菲更容易被微生物利用.生物炭中大量的营养元素、有效水、完善的孔道结构和较大的比表面积为微生物提供了良好的栖息场所.此外,生物炭孔径可保护微生物免受捕食者或竞争者的影响,改善土壤理化性质,尤其是增加土壤持水能力,改变土壤体积密度从而促进气体运输,这些改变可能会提高微生物活性[36](如图 1(b)所示).Cheng等[42]发现加入生物炭后降低了土豆对苯醚甲环唑植物吸收,但增加了土壤中微生物对其的降解.添加生物炭会增加土壤细菌群体中Sphingomonadaceae和Pseudomonadaceae的相对丰度,促进土壤微生物对污染物的降解.Kong等[43]发现在添加生物炭后,土壤中细菌群体多样性略有下降,但PAHs降解菌的丰度增加,促进PAHs生物降解.
此外,生物炭由于含有表面氧化还原活性官能团或导电石墨结构,具有电子介导能力,促进电子从电活性微生物(例如Shewanella oneidensis MR-1、Geobacter sulfurreducens)转移到有机污染物,加快污染物降解[44](如图 1(c)所示).Tong等[45]的报道中,生物炭添加到土壤中后,作为电子介体加速土壤有机质到细菌之间的电子转移,刺激了脱氯菌和铁还原菌的活性,增加了土壤微生物群体中脱氯菌和铁还原菌微生物丰度,从而加快五氯酚还原脱氯.同时,添加生物炭可以促进Fe(Ⅲ)还原,生成的Fe(Ⅱ)作为还原剂加快五氯酚化学转化.Chen等[26]也发现生物炭在促进沉淀中四溴联苯醚微生物脱溴中起到3个作用.首先,生物炭促进有机卤化物呼吸菌生长,改变了微生物群体组成,增加了铁还原菌、硫还原菌和脱卤菌微生物的丰度.其次,生物炭作为电子介体加速脱氯微生物到2, 2′, 4, 4′-四溴联苯醚电子转移.最后,添加生物炭促进Fe(Ⅱ)生成,促进四溴联苯醚非生物转化.
总体来说,生物炭可以通过对有机污染物的吸附作用、对微生物的促进作用以及本身电子介导作用影响有机污染物生物可利用性.生物炭对有机污染物降解的促进或抑制取决于哪种作用占主导.目前,关于此方面的研究仍停留在定性解释阶段,很少有定量分析在某个环境下生物炭各个作用对有机污染物生物可利用性影响的贡献率.不同制备条件下获得的生物炭可能产生不同的影响.随着生物炭热解温度增加,其比表面积和芳香性增加,但表面官能团含量降低.一般来说,高温生物炭由于具有较高的比表面积和芳香性,对于疏水性有机污染物有较高的吸附能力[46].低温生物炭含更多易被微生物利用的有机质,因此,对微生物生长有更明显的促进作用[47-48].同时,对于生物炭电子特性,中温和高温生物炭具有较高的电子转移能力[48].目前,大部分研究都是针对生物炭热解温度对某一作用的影响,没有将三者综合考虑.此外,在土壤修复中,生物炭对有机污染物生物降解的影响既取决于生物炭本身结构特征,例如生物炭比表面积、表面官能团、芳香碳结构等,也受生物炭剂量、老化时间和微生物特征等因素的影响[7, 49-51].Song等[50]发现加入0.1%生物炭对六氯碳微生物降解没有影响,但加入5%生物炭后,在前一个月促进了六氯苯还原脱氯,之后产生抑制作用.促进作用可能是由生物炭氧化还原活性造成的.但随着时间的延长,生物炭颗粒发生老化,其氧化还原活性部位被掩盖.抑制作用可能是由于生物炭的吸附作用导致生物可利用性降低.同理,对比Zhu等[7]和Chen等[51]的实验,生物炭对四溴联苯醚脱溴呈现不同效果.这是因为Zhu等[7]发现反应时间长,老化作用占主导地位,而Chen等[51]的实验中添加生物炭后污染物的沉淀被迅速用于降解实验,且在前者的实验中用的是降解菌株,而后者使用的是完整的本土微生物群体.因此,在具体研究某种生物炭对有机污染物生物降解的影响时,要综合考虑多方面因素.
2.2 堆肥添加剂堆肥添加剂是有机废物堆肥化过程的产物,由于其经济性和可持续性,在农业施肥中被广泛应用[52-53].有研究表明[19, 21, 54],堆肥添加剂在污染土壤修复过程中也起到重要的作用.
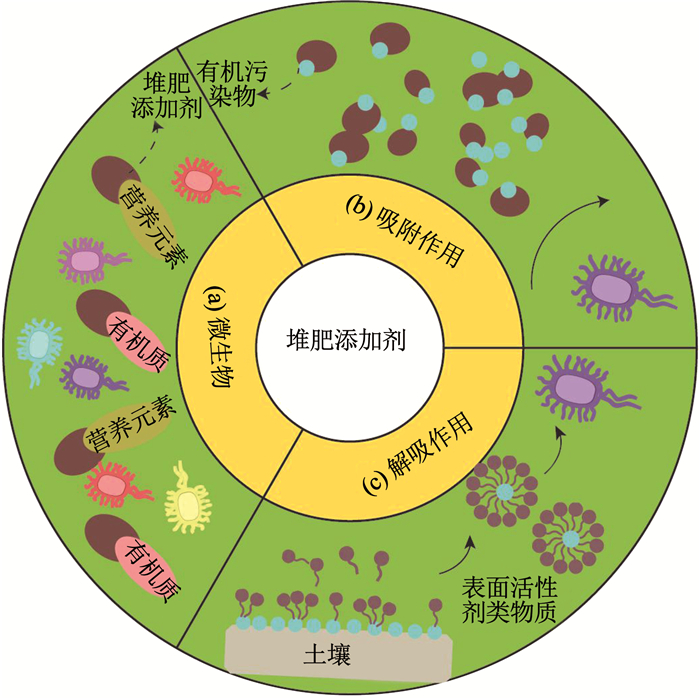
Bastida等[55]研究发现在自然土壤中碳氢化合物的降解并不明显,而加入堆肥产物后其降解率在50 d后达到了88%.这可能是由于堆肥添加剂能够提高土壤微生物的数量和活性.Wallisch等[56]研究发现加入堆肥产物可以促进烷烃降解菌的生长,从而提高烷烃降解效率.Baldantoni等[57]也报道加入堆肥产物后PAHs的降解速率和过氧化物酶活性增高.堆肥产物中富含大量活性有机质和营养元素,可被微生物利用,从而增加了微生物丰度(如图 2(a)所示).同时,由于堆肥材料具有较高的持水能力,且能提高土壤结构稳定性,增加土壤通透性,也有利于微生物生长.
|
图 2 堆肥添加剂影响有机污染物生物可利用性机理 Fig. 2 Effect of compost on the bioavailability of organic pollutants |
堆肥添加剂对有机污染物生物可利用性的影响是多方面的[58].Puglisi等[59]报道,排除微生物作用后,堆肥产物中的大量有机质增强了土壤对菲的吸附作用,导致其生物可利用性降低(如图 2(b)所示).堆肥基质中的木质素/纤维素残留物对PAHs的固定起到重要的作用[60].然而,考虑微生物的作用,堆肥添加剂提高了本土微生物活性,同时也引入菲降解微生物,因此促进了菲降解[59].Sigmund等[61]发现加入10%堆肥产物后,即使PAHs吸附系数增加了10倍,其降解速率也增加了2倍.这可能是由于微生物活性的提高和外源微生物群体的引入对PAHs降解有促进作用,且此实验中堆肥有机质对PAHs的吸附是可逆的,对微生物降解没有显著抑制作用.不同于生物炭,堆肥有机质富含溶解性有机物,对污染物的固定和释放有多重作用.有研究报道堆肥产物中的水分可提取态有机物质能增加PAHs表观溶解度,且易吸附在细胞表面,使得和水分可提取态有机物质相互作用的污染物更容易被微生物吸收和降解[62].Wu等[60]报道,反应初始,在柴油污染土壤中加入堆肥产物后由于强吸附作用使得PAHs去除量降低89%,但随着时间的推移,PAHs去除量比未添加土壤增加了2倍,其中30%是由于解吸程度提高.堆肥产物中的腐殖酸类化合物的作用类似生物表面活性剂,它可以降低表面张力,并通过形成胶束促进PAHs的解吸[60](如图 2(c)所示).土壤中的非水相液体,例如焦油,是有机污染物迁移的主要阻力.堆肥产物与土壤混合后,形成一个适合传质的微环境,破坏该非水相液体界面的表面阻力,从而促进有机污染物迁移[19].因此,堆肥有机质对有机污染物生物可利用影响也是多方面的.
总的来说,堆肥添加剂对有机污染生物可利用性有3个方面的作用.一方面,堆肥产物中富含营养元素和有机质,促进本土微生物活性,同时也引入一些活性降解菌,促进污染物降解;另一方面,引入有机质加强了土壤对有机污染物的吸附;此外,堆肥产物中的溶解性有机质能提高污染物溶解性,促进污染物解吸,从而有利于微生物对有机污染物的吸收.堆肥产物的特征(营养元素、有机质特征和微生物)主要受堆肥原料、堆肥温度和腐熟时间等因素的影响.这些因素也会影响堆肥产物对有机污染物生物可利用性作用效果.例如, Plaza等[63]研究了不同堆肥阶段有机质提取的腐殖酸对PAHs吸附作用力.与土壤腐殖酸相比,堆肥初始阶段腐殖酸对PAHs有更大的吸附作用力,加入土壤后促进了PAHs吸附.随着堆肥的进行, 腐殖酸结构和化学组成发生了显著变化,芳香性和极性增加,因此,对PAHs的吸附作用力减弱,其性质类似于土壤腐殖酸,有利于PAHs被微生物降解.目前关于堆肥产物特征对有机污染物生物修复影响的研究很少,需要更多的研究.
2.3 表面活性剂表面活性剂是一类同时含有亲水性和疏水性结构的两亲性化学物质,其独特的分子结构可增加污染物表观溶解度,尤其是疏水性有机污染物.大量研究表明,表面活性剂能促进土壤中有机污染物生物降解[6, 64-67].辛烷质量分数分别为700和70 000 mg/kg时,向土壤中添加表面活性剂使得辛烷降解效率分别增加57.4%和38.8%[66].Bezza和Chirwa[6]也发现加入生物表面活性剂后土壤中PAHs降解达86.5%,而不添加情况下降解率只有57%.表面活性剂通过影响污染物在溶液中分散或微生物表面特征,从而增加污染物生物可利用性[68-70].
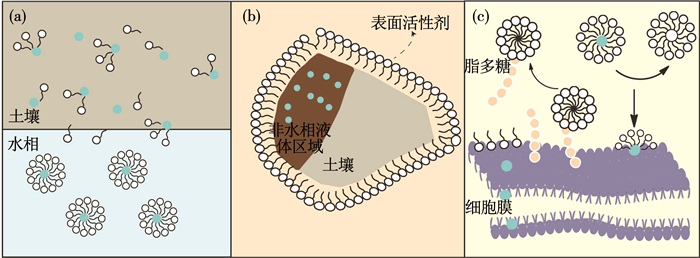
表面活性剂对污染物增溶作用是其增加有机污染物生物可利用性最重要的途径.当表面活性剂浓度高于临界胶束浓度,胶束疏水性核形成微观疏水环境,为有机污染物提供额外吸附位点,而亲水性尾部允许胶束溶于水相,从而增加了污染物水相浓度[64](如图 3(a)所示).Singh等[71]证明添加表面活性剂使得毒死蜱水相溶解度增加超过10倍,且其生物降解提高了30%.
|
图 3 表面活性剂影响有机污染物生物可利用性机理 Fig. 3 Effect of surfactant on the bioavailability of organic pollutants |
除了胶束增溶作用,表面活性剂可能改变污染物基质,这通常发生在表面活性剂浓度低于临界胶束浓度的情况.相对疏水的非离子型表面活性剂可吸附在土壤中的非水相液体区域(煤焦油、杂酚油和石油等),使其有利于污染物解吸或微生物与污染物接触[72](如图 3(b)所示).在Yeom等[73]的实验中,表面活性剂促进PAHs解吸主要通过增加基质的扩散性,而胶束溶解作用是次要的.表面活性剂具有两亲性质,容易在表面和界面处聚集,从而降低表面和界面的张力,增加污染物移动性[74].Cecotti等[75]也报道,在传统生物处理后的土壤中,加入低于临界胶束浓度的表面活性剂同样促进了PAHs解吸,而该情况下胶束溶解作用可以忽略,因此,这种促进作用可能是通过增加PAHs在基质中扩散性.
除了和污染物以及污染介质发生相互作用,表面活性剂可能影响土壤微生物,通过增加微生物和目标污染物的接触,从而提高生物可利用性(如图 3(c)所示).Lanzon和Brown[76]证明非离子表面活性剂Brij 30可吸附在细菌细胞表面形成半胶团,使细菌表面吸附态菲增加2倍.表面活性剂对微生物表面特征的影响主要体现在细胞界面疏水性和细胞膜流动性.表面活性剂吸附在细胞表面,改变细胞表面疏水性,而细胞表面疏水性影响细菌聚集状态和生物膜的形成[77].微生物对疏水性基质的黏附性也取决于细胞表面疏水性.Zhang和Zhu[78]的实验中,微生物细胞表面主要是亲水性,表面活性剂占据细胞亲水性位点,其疏水性尾部存在于环境中,导致细胞表面疏水性位点增加,从而促进了Klebsiella oxytoca PYR-1和芘之间的吸附.同时,Li和Zhu[79]也报道土壤中添加50 mg/L吐温80或十二烷基苯磺酸钠后,细胞表面疏水性增加了19.8%~25.2%,菲生物降解率增加了8.9%~17.2%.这是由于表面活性剂洗涤特征和其对膜表面脂多糖溶解作用促进了细胞表面脂多糖释放,使得细胞外膜暴露在水相,导致细胞表面疏水性增加,促进PAHs被微生物吸收.此外,表面活性剂也可增加细胞膜流动性.Li和Zhu[80]研究发现,添加表面活性剂后菲降解菌细胞膜脂肪酸组成发生改变,不饱和脂肪酸量增加,膜流动性增强,促进菲从胞外基质分配到细胞表面.Li等[81]进一步研究基因水平上表面活性剂对生物降解过程的提高.表面活性剂可以降低细胞膜中脂磷壁酸的含量,通过调节相关酶基因表达水平来增加细胞疏水性和膜流动性,从而增加了不饱和脂肪酸含量,进一步促进菲分配和跨膜迁移.除了对微生物活性产生影响,表面活性剂还可能改变土壤微生物群体组成[75],但这种变化对污染物降解的影响机理尚不清楚,需要进一步研究.
因此,表面活性剂能增加有机污染物表观溶解度,提高基质扩散性,改变微生物细胞表面疏水性和膜通透性,从而增加有机污染物生物可利用性.表面活性剂作用效果受表面活性剂种类和浓度、参与的微生物种类的影响.Patel等[82]发现在非离子表面活性剂Tween 80存在下,菲的降解率由86%提高到100%,但在十二烷基硫酸钠和十六烷基三甲基溴化铵存在下,菲的降解率分别降低到45%和19%,这种抑制作用可能是由于该表面活性剂对微生物生长的抑制.也有研究发现十二烷基硫酸钠抑制Pseudoxanthomonas sp.DMVP2对菲生物降解,接近64%,但促进了混合菌株DAK11对菲的降解,接近88.79%[83-84].由此可见,不同种类的微生物在同一表面活性剂作用下对有机污染物的去除效果不同.之前的研究也发现[85],鼠李糖脂的溶解作用增加了十六烷对P.aeruginosa生物可利用性,但降低了对P.putida生物可利用性.Pseudomonas putida CICC 20575为非鼠李糖脂分泌菌,鼠李糖脂-十六烷无法被细胞膜吸收,为了进入核中的十六烷,必须先破坏外层表面活性剂,因此,鼠李糖脂的存在抑制了生物可利用性.表面活性剂对微生物的毒性效应,微生物优先利用表面活性剂而非目标污染物,或者表面活性剂阻碍溶解于胶束内的有机污染物和微生物接触等,都可能抑制有机污染物生物降解.在利用表面活性剂作为土壤修复时,要考虑到表面活性剂生物毒性、环境持久性以及合适的添加浓度,使其能最大限度地提高有机污染物生物可利用性,促进有机污染物生物降解.
3 结论低生物可利用性是限制有机污染物生物降解的主要因素,因此,探究生物修复方法对生物可利用性影响是很重要的.土壤改良剂对生物可利用性影响主要包括以下几个方面:影响污染物在土壤中吸附和解吸,污染物和微生物之间传质以及微生物丰度和活性.理解土壤改良剂对有机污染物生物可利用性的影响机理有利于寻找合适的土壤改良剂.目前,关于该3种土壤改良剂对有机污染物生物可利用性的作用机理研究多停留在定性描述阶段,很少有定量分析,有待于进一步研究,具体表现为:
1) 生物炭对有机污染物生物可利用性影响机理多是现象描述,关于生物炭3种作用机制对有机污染物生物可利用性影响的贡献率少有研究.此外,生物炭作用效果受其本身结构特征和应用条件的影响,但这些影响因素对有机污染物生物可利用性影响缺乏全面系统的研究,建议后续研究可以从生物炭对有机污染物吸附作用、对微生物促进作用以及本身电子介导作用变化这3个方面进行分析.
2) 通过堆肥过程控制可调控堆肥产物的特征,但目前少有研究堆肥产物不同特征是否对土壤有机污染物生物可利用性产生不同影响.
3) 表面活性剂用于土壤修复的目的是增加有机污染物生物可利用性,但受表面活性剂种类和浓度、参与的微生物种类等因素影响,表面活性剂可能降低有机污染物生物可利用性.
| [1] |
CHENG M, ZENG G M, HUANG D L, et al. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review[J]. Chemical Engineering Journal, 2016, 284: 582. DOI:10.1016/j.cej.2015.09.001 |
| [2] |
GONG X M, HUANG D L, LIU Y G, et al. Remediation of contaminated soils by biotechnology with nanomaterials: Bio-behavior, applications, and perspectives[J]. Critical Reviews in Biotechnology, 2018, 38(3): 455. DOI:10.1080/07388551.2017.1368446 |
| [3] |
LIU S H, ZENG G M, NIU Q Y, et al. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review[J]. Bioresource Technology, 2017, 224: 25. DOI:10.1016/j.biortech.2016.11.095 |
| [4] |
LIU Z F, LIU Y J, ZENG G M, et al. Application of molecular docking for the degradation of organic pollutants in the environmental remediation: A review[J]. Chemosphere, 2018, 203: 139. DOI:10.1016/j.chemosphere.2018.03.179 |
| [5] |
ZENG G M, CHEN M, ZENG Z. Risks of neonicotinoid pesticides[J]. Science, 2013, 340(6139): 1403. DOI:10.1126/science.340.6139.1403-a |
| [6] |
BEZZA F A, CHIRWA E M N. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil[J]. Chemosphere, 2016, 144: 635. DOI:10.1016/j.chemosphere.2015.08.027 |
| [7] |
ZHU B, XIA X, WU S, et al. Microbial bioavailability of 2, 2′, 4, 4′-Tetrabromodiphenyl ether (BDE-47) in natural sediments from major rivers of China[J]. Chemosphere, 2016, 153: 386. DOI:10.1016/j.chemosphere.2016.03.050 |
| [8] |
YU Z, ZENG G M, CHEN Y N, et al. Effects of inoculation with Phanerochaete chrysosporium on remediation of pentachlorophenol-contaminated soil waste by composting[J]. Process Biochemistry, 2011, 46(6): 1285. DOI:10.1016/j.procbio.2011.02.018 |
| [9] |
ZENG G M, YU Z, CHEN Y N, et al. Response of compost maturity and microbial community composition to pentachlorophenol (PCP)-contaminated soil during composting[J]. Bioresource Technology, 2011, 102(10): 5905. DOI:10.1016/j.biortech.2011.02.088 |
| [10] |
REN X Y, ZENG G M, TANG L, et al. Effect of exogenous carbonaceous materials on the bioavailability of organic pollutants and their ecological risks[J]. Soil Biology and Biochemistry, 2018, 116: 70. DOI:10.1016/j.soilbio.2017.09.027 |
| [11] |
CHEN Y N, MA S, LI Y P, et al. Microbiological study on bioremediation of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) contaminated soil by agricultural waste composting[J]. Applied Microbiology and Biotechnology, 2016, 100(22): 9709. DOI:10.1007/s00253-016-7798-8 |
| [12] |
HUANG D L, QIN X M, XU P, et al. Composting of 4-nonylphenol-contaminated river sediment with inocula of Phanerochaete chrysosporium[J]. Bioresource Technology, 2016, 221: 47. DOI:10.1016/j.biortech.2016.08.104 |
| [13] |
XU P, LAI C, ZENG G M, et al. Enhanced bioremediation of 4-nonylphenol and cadmium co-contaminated sediment by composting with Phanerochaete chrysosporium inocula[J]. Bioresource Technology, 2018, 250: 625. DOI:10.1016/j.biortech.2017.11.069 |
| [14] |
ZHAO C H, YAN M, ZHONG H, et al. Biodegradation of polybrominated diphenyl ethers and strategies for acceleration: A review[J]. International Biodeterioration & Biodegradation, 2018, 129: 23. DOI:10.1016/j.ibiod.2017.12.010 |
| [15] |
REN X Y, ZENG G M, TANG L, et al. Sorption, transport and biodegradation: An insight into bioavailability of persistent organic pollutants in soil[J]. Science of the Total Environment, 2018, 610/611: 1154. DOI:10.1016/j.scitotenv.2017.08.089 |
| [16] |
CHEN B, DING J. Biosorption and biodegradation of phenanthrene and pyrene in sterilized and unsterilized soil slurry systems stimulated by Phanerochaete chrysosporium[J]. Journal of Hazardous Materials, 2012, 229/230(3): 159. DOI:10.1016/j.jhazmat.2012.05.090 |
| [17] |
BRICEÑO G, PALMA G, DURÁN N. Influence of organic amendment on the biodegradation and movement of pesticides[J]. Critical Reviews in Environmental Science and Technology, 2007, 37(3): 233. DOI:10.1080/10643380600987406 |
| [18] |
DING Y, LIU Y G, LIU S B, et al. Biochar to improve soil fertility: A review[J]. Agronomy for Sustainable Development, 2016, 36(2): 36. DOI:10.1007/s13593-016-0372-z |
| [19] |
KÄSTNER M, MILTNER A. Application of compost for effective bioremediation of organic contaminants and pollutants in soil[J]. Applied Microbiology & Biotechnology, 2016, 100(8): 1. DOI:10.1007/s00253-016-7378-y |
| [20] |
ZENG Z, LIU Y, ZHONG H, et al. Mechanisms for rhamnolipids-mediated biodegradation of hydrophobic organic compounds[J]. Science of the Total Environment, 2018, 634: 1. DOI:10.1016/j.scitotenv.2018.03.349 |
| [21] |
CHEN M, XU P, ZENG G M, et al. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs[J]. Biotechnology Advances, 2015, 33(6): 745. DOI:10.1016/j.biotechadv.2015.05.003 |
| [22] |
CHENG M, ZENG G M, HUANG D L, et al. Advantages and challenges of Tween 80 surfactant-enhanced technologies for the remediation of soils contaminated with hydrophobic organic compounds[J]. Chemical Engineering Journal, 2017, 314: 98. DOI:10.1016/j.cej.2016.12.135 |
| [23] |
WU H P, LAI C, ZENG G M. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: A review[J]. Critical Reviews in Biotechnology, 2017, 37(6): 754. DOI:10.1080/07388551.2016.1232696 |
| [24] |
YE S J, ZENG G M, WU H P, et al. Biological technologies for the remediation of co-contaminated soil[J]. Critical Reviews in Biotechnology, 2017, 37(8): 1062. DOI:10.1080/07388551.2017.1304357 |
| [25] |
EHLERS L J, LUTHY R G. Peer reviewed: Contaminant bioavailability in soil and sediment[J]. Environmental Science & Technology, 2003, 37(15): 295A. |
| [26] |
RONCEVIC S, SPASOJEVIC J, MALETIC S, et al. Assessment of the bioavailability and phytotoxicity of sediment spiked with polycyclic aromatic hydrocarbons[J]. Environmental Science & Pollution Research, 2016, 23(4): 1. DOI:10.1007/s11356-015-5566-4 |
| [27] |
SEMPLE K T, DOICK K J, JONES K C, et al. Peer reviewed: Defining bioavailability and bioaccessibility of contaminated soil and sediment is complicated[J]. Environmental Science & Technology, 2004, 38(12): 228A. |
| [28] |
KICKHAM P, OTTON S V, MOORE M M, et al. Relationship between biodegradation and sorption of phthalate esters and their metabolites in natural sediments[J]. Environmental Toxicology Chemistry, 2012, 31(8): 1730. DOI:10.1002/etc.1903 |
| [29] |
SEMPLE K T, DOICK K J, WICK L Y, et al. Microbial interactions with organic contaminants in soil: Definitions, processes and measurement[J]. Environmental Pollution, 2007, 150(1): 166. DOI:10.1002/etc.1903 |
| [30] |
MEGHARAJ M, RAMAKRISHNAN B, VENKATESWARLU K, et al. Bioremediation approaches for organic pollutants: A critical perspective[J]. Environment International, 2011, 37(8): 1362. DOI:10.1016/j.envint.2011.06.003 |
| [31] |
WU S, HE H, INTHAPANYA X, et al. Role of biochar on composting of organic wastes and remediation of contaminated soils: A review[J]. Environmental Science & Pollution Research International, 2017, 24(20): 1. DOI:10.1007/s11356-017-9168-1 |
| [32] |
DING Y, LIU Y G, LIU S B, et al. Potential benefits of biochar in agricultural soils: A review[J]. Pedosphere, 2017, 27(4): 645. DOI:10.1016/S1002-0160(17)60375-8 |
| [33] |
TANG L, YU J F, PANG Y, et al. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal[J]. Chemical Engineering Journal, 2018, 336: 160. DOI:10.1016/j.cej.2017.11.048 |
| [34] |
HUANG M, LI Z W, LUO N L, et al. Application potential of biochar in environment: Insight from degradation of biochar-derived DOM and complexation of DOM with heavy metals[J]. Science of the Total Environment, 2019, 646: 220. DOI:10.1016/j.scitotenv.2018.07.282 |
| [35] |
TAN X F, LIU S B, LIU Y G, et al. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage[J]. Bioresource Technology, 2017, 227: 359. DOI:10.1016/j.biortech.2016.12.083 |
| [36] |
ZHU X, CHEN B, ZHU L, et al. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review[J]. Environmental Pollution, 2017, 227: 98. DOI:10.1016/j.envpol.2017.04.032 |
| [37] |
YANG Y, SHENG G, HUANG M. Bioavailability of diuron in soil containing wheat-straw-derived char[J]. Science of the Total Environment, 2006, 354(2): 170. DOI:10.1016/j.scitotenv.2005.01.026 |
| [38] |
MARCHAl G, SMITH K E, REIN A, et al. Impact of activated carbon, biochar and compost on the desorption and mineralization of phenanthrene in soil[J]. Environmental Pollution, 2013, 181(6): 200. DOI:10.1016/j.envpol.2013.06.026 |
| [39] |
ZHU B, WU S, XIA X, et al. Effects of carbonaceous materials on microbial bioavailability of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) in sediments[J]. Journal of Hazardous Materials, 2016, 312: 216. DOI:10.1016/j.jhazmat.2016.03.065 |
| [40] |
XIA X, LI Y, ZHOU Z, et al. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium[J]. Chemosphere, 2010, 78(11): 1329. DOI:10.1016/j.chemosphere.2010.01.007 |
| [41] |
RHODES A H, RIDING M J, MCALLISTER L E, et al. Influence of activated charcoal on desorption kinetics and biodegradation of phenanthrene in soil[J]. Environmental Science & Technology, 2012, 46(22): 12445. DOI:10.1021/es3025098 |
| [42] |
CHENG J, LEE X, GAO W, et al. Effect of biochar on the bioavailability of difenoconazole and microbial community composition in a pesticide-contaminated soil[J]. Applied Soil Ecology, 2017, 121: 185. DOI:10.1016/j.apsoil.2017.10.009 |
| [43] |
KONG L, GAO Y, ZHOU Q, et al. Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy[J]. Journal of Hazardous Materials, 2017, 343: 276. DOI:10.1016/j.jhazmat.2017.09.040 |
| [44] |
YU L, YUAN Y, TANG J, et al. Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens[J]. Scientific Reports, 2015, 5: 16221. DOI:10.1038/srep16221 |
| [45] |
TONG H, HU M, LI F B, et al. Biochar enhances the microbial and chemical transformation of pentachlorophenol in paddy soil[J]. Soil Biology & Biochemistry, 2014, 70(2): 142. DOI:10.1016/j.soilbio.2013.12.012 |
| [46] |
TANG J, ZHU W, KOOKANA R, et al. Characteristics of biochar and its application in remediation of contaminated soil[J]. Journal of Bioscience and Bioengineering, 2013, 116(6): 653. DOI:10.1016/j.jbiosc.2013.05.035 |
| [47] |
LUO Y, DUNGAIT J A, ZHAO X, et al. Pyrolysis temperature during biochar production alters its subsequent utilization by microorganisms in an acid arable soil[J]. Land Degradation & Development, 2018, 29(7): 2183. DOI:10.1002/ldr.2846 |
| [48] |
ZHU X, CHEN B, ZHU L, et al. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review[J]. Environmental Pollution, 2017, 227: 98. DOI:10.1016/j.envpol.2017.04.032 |
| [49] |
CHENG G, SUN M, LU J, et al. Role of biochar in biodegradation of nonylphenol in sediment: Increasing microbial activity versus decreasing bioavailability[J]. Scientific Reports, 2017, 7(1): 4726. DOI:10.1038/s41598-017-04787-2 |
| [50] |
SONG Y, BIAN Y, WANG F, et al. Effects of biochar on dechlorination of hexachlorobenzene and the bacterial community in paddy soil[J]. Chemosphere, 2017, 186: 116. DOI:10.1016/j.chemosphere.2017.07.139 |
| [51] |
CHEN J, WANG C, PAN Y, et al. Biochar accelerates microbial reductive debromination of 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47) in anaerobic mangrove sediments[J]. Journal of Hazardous Materials, 2017, 341: 177. DOI:10.1016/j.jhazmat.2017.07.063 |
| [52] |
LUO Y, LIANG J, ZENG G M, et al. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects[J]. Waste Management, 2018, 71: 109. DOI:10.1016/j.wasman.2017.09.023 |
| [53] |
WU H P, ZENG G M, LIANG J, et al. Responses of bacterial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil[J]. Applied Microbiology & Biotechnology, 2016, 100(19): 8583. DOI:10.1007/s00253-016-7614-5 |
| [54] |
HUANG M, ZHU Y, LI Z W, et al. Compost as a soil amendment to remediate heavy metal-contaminated agricultural soil: Mechanisms, efficacy, problems, and strategies[J]. Water Air and Soil Pollution, 2016, 227(10): 359. DOI:10.1007/s11270-016-3068-8 |
| [55] |
BASTIDA F, JEHMLICH N, LIMA K, et al. The ecological and physiological responses of the microbial community from a semiarid soil to hydrocarbon contamination and its bioremediation using compost amendment[J]. Journal of Proteomics, 2015, 135(7): 162. DOI:10.1016/j.jprot.2015.07.023 |
| [56] |
WALLISCH S, GRIL T, DONG X, et al. Effects of different compost amendments on the abundance and composition of alkB harboring bacterial communities in a soil under industrial use contaminated with hydrocarbons[J]. Frontiers in Microbiology, 2014, 5(96): 96. DOI:10.3389/fmicb.2014.00096 |
| [57] |
BALDANTONI D, MORELLI R, BELLINO A, et al. Anthracene and benzo(a)pyrene degradation in soil is favoured by compost amendment: Perspectives for a bioremediation approach[J]. Journal of Hazardous Materials, 2017, 339: 395. DOI:10.1016/j.jhazmat.2017.06.043 |
| [58] |
REN X Y, ZENG G M, TANG L, et al. The potential impact on the biodegradation of organic pollutants from composting technology for soil remediation[J]. Waste Management, 2018, 72: 138. DOI:10.1016/j.wasman.2017.11.032 |
| [59] |
PUGLISI E, CAPPA F, FRAGOULIS G, et al. Bioavailability and degradation of phenanthrene in compost amended soils[J]. Chemosphere, 2007, 67(3): 548. DOI:10.1016/j.chemosphere.2006.09.058 |
| [60] |
WU G, KECHAVARZI C, LI X, et al. Influence of mature compost amendment on total and bioavailable polycyclic aromatic hydrocarbons in contaminated soils[J]. Chemosphere, 2013, 90(8): 2240. DOI:10.1016/j.chemosphere.2012.10.003 |
| [61] |
SIGMUND G, POYNTNER C, PIÑAR G, et al. Influence of compost and biochar on microbial communities and the sorption/degradation of PAHs and NSO-substituted PAHs in contaminated soils[J]. Journal of Hazardous Materials, 2017, 345: 107. DOI:10.1016/j.jhazmat.2017.11.010 |
| [62] |
KOBAYASHI T, MURAI Y, TATSUMI K, et al. Biodegradation of polycyclic aromatic hydrocarbons by Sphingomonas sp. enhanced by water-extractable organic matter from manure compost[J]. Science of the Total Environment, 2009, 407(22): 5805. DOI:10.1016/j.scitotenv.2009.06.041 |
| [63] |
PLAZA C, XING B, FERNÁNDEZ J M, et al. Binding of polycyclic aromatic hydrocarbons by humic acids formed during composting[J]. Environmental Pollution, 2009, 157(1): 257. DOI:10.1016/j.envpol.2008.07.016 |
| [64] |
MAO X, JIANG R, XIAO W, et al. Use of surfactants for the remediation of contaminated soils: A review[J]. Journal of Hazardous Materials, 2015, 285: 419. DOI:10.1016/j.scitotenv.2009.06.041 |
| [65] |
ZHONG H, LIU Y, LIU Z F, et al. Degradation of pseudo-solubilized and mass hexadecane by a Pseudomonas aeruginosa with treatment of rhamnolipid biosurfactant[J]. International Biodeterioration & Biodegradation, 2014, 94(94): 152. DOI:10.1016/j.ibiod.2014.07.012 |
| [66] |
MOLDES A B, PARADELO R, RUBINOS D, et al. Ex situ treatment of hydrocarbon-contaminated soil using biosurfactants from lactobacillus pentosus[J]. Journal of Agricultural & Food Chemistry, 2011, 59(17): 9443. DOI:10.1021/jf201807r |
| [67] |
CHENG M, ZENG G M, HUANG D L, et al. Tween 80 surfactant-enhanced bioremediation: Toward a solution to the soil contamination by hydrophobic organic compounds[J]. Critical Reviews in Biotechnology, 2018, 38(1): 17. DOI:10.1080/07388551.2017.1311296 |
| [68] |
YUAN X Z, REN F Y, ZENG G M, et al. Adsorption of surfactants on a Pseudomonas aeruginosa strain and the effect on cell surface lypohydrophilic property[J]. Applied Microbiology & Biotechnology, 2007, 76(5): 1189. DOI:10.1007/s00253-007-1080-z |
| [69] |
SHAO B B, LIU Z F, ZHONG H, et al. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review[J]. Microbiological Research, 2017, 200: 33. DOI:10.1016/j.micres.2017.04.005 |
| [70] |
ZHONG H, WANG Z Q, LIU Z F, et al. Degradation of hexadecane by Pseudomonas aeruginosa with the mediation of surfactants: Relation between hexadecane solubilization and bioavailability[J]. International Biodeterioration & Biodegradation, 2016, 115: 141. DOI:10.1016/j.ibiod.2016.08.008 |
| [71] |
SINGH P, SAINI H S, RAJ M. Rhamnolipid mediated enhanced degradation of chlorpyrifos by bacterial consortium in soil-water system[J]. Ecotoxicology & Environmental Safety, 2016, 134: 156. DOI:10.1016/j.ecoenv.2016.07.020 |
| [72] |
ADRION A C, NAKAMURA J, SHEA D, et al. Screening nonionic surfactants for enhanced biodegradation of polycyclic aromatic hydrocarbons remaining in soil after conventional biological treatment[J]. Environmental Science & Technology, 2016, 50(7): 3838. DOI:10.1021/acs.est.5b05243 |
| [73] |
YEOM I T, GHOSH M M, COX C D. Kinetic aspects of surfactant solubilization of soil-bound polycyclic aromatic hydrocarbons[J]. Environmental Science & Technology, 1996, 30(5): 1589. DOI:10.1021/es950567t |
| [74] |
MULLIGAN C N, YONG R N, GIBBS B F. Surfactant-enhanced remediation of contaminated soil: A review[J]. Engineering Geology, 2001, 60(1): 371. DOI:10.1016/S0013-7952(00)00117-4 |
| [75] |
CECOTTI M, COPPOTELLI B M, MORA V C, et al. Efficiency of surfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbon-contaminated soil: Link with bioavailability and the dynamics of the bacterial community[J]. Science of the Total Environment, 2018, 634: 224. DOI:10.1016/j.scitotenv.2018.03.303 |
| [76] |
LANZON J B, BROWN D G. Partitioning of phenanthrene into surfactant hemi-micelles on the bacterial cell surface and implications for surfactant-enhanced biodegradation[J]. Water Research, 2013, 47(13): 4612. DOI:10.1016/j.watres.2013.04.062 |
| [77] |
LIU Z F, ZENG Z T, ZENG G M, et al. Influence of rhamnolipids and Triton X-100 on adsorption of phenol by Penicillium simplicissimum[J]. Bioresource Technology, 2012, 110: 468. DOI:10.1016/j.biortech.2012.01.092 |
| [78] |
ZHANG D, ZHU L. Effects of Tween 80 on the removal, sorption and biodegradation of pyrene by Klebsiella oxytoca PYR-1[J]. Environmental Pollution, 2012, 164(1): 169. DOI:10.1016/j.envpol.2012.01.036 |
| [79] |
LI F, ZHU L. Effect of surfactant-induced cell surface modifications on electron transport system and catechol 1, 2-dioxygenase activities and phenanthrene biodegradation by Citrobacter sp. SA01[J]. Bioresource Technology, 2012, 123(2): 42. DOI:10.1016/j.biortech.2012.07.059 |
| [80] |
LI F, ZHU L. Surfactant-modified fatty acid composition of Citrobacter sp. SA01 and its effect on phenanthrene transmembrane transport[J]. Chemosphere, 2014, 107: 58. DOI:10.1016/j.chemosphere.2014.03.016 |
| [81] |
LI F, ZHU L, WANG L, et al. Gene expression of an Arthrobacter in surfactant-enhanced biodegradation of a hydrophobic organic compound[J]. Environmental Science & Technology, 2015, 49(6): 3698. DOI:10.1021/es504673j |
| [82] |
PATEL V, PATEL J, MADAMWAR D. Biodegradation of phenanthrene in bioaugmented microcosm by consortium ASP developed from coastal sediment of Alang-Sosiya ship breaking yard[J]. Marine Pollution Bulletin, 2013, 74(1): 199. DOI:10.1016/j.marpolbul.2013.07.001 |
| [83] |
PATEL V, CHETURVEDULA S, MADAMWAR D. Phenanthrene degradation by Pseudoxanthomonas sp. DMVP2 isolated from hydrocarbon contaminated sediment of Amlakhadi canal, Gujarat, India[J]. Journal of Hazardous Materials, 2012, 201/202: 43. DOI:10.1016/j.jhazmat.2011.11.002 |
| [84] |
PATEL A B, MAHALA K, JAIN K, et al. Development of mixed bacterial cultures DAK11 capable for degrading mixture of polycyclic aromatic hydrocarbons (PAHs)[J]. Bioresource Technology, 2018, 253: 288. DOI:10.1016/j.biortech.2018.01.049 |
| [85] |
LIU Y, ZENG G M, ZHONG H, et al. Effect of rhamnolipid solubilization on hexadecane bioavailability: Enhancement or reduction?[J]. Journal of Hazardous Materials, 2017, 322: 394. DOI:10.1016/j.jhazmat.2016.10.025 |
 2020, Vol. 52
2020, Vol. 52


