2. State Key Laboratory of Urban Water Resource and Environment, School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin 150090, China
Excessive use of nitrogenous fertilizers in agricultural activities and inappropriate treatment of wastewaters resulted in nitrate concentration in surface and ground waters to exceed maximum allowable limits for the drinking water[1]. Therefore, contamination of water by nitrate is increasing worldwide and nitrate level in natural waters becomes an important indicator of water quality. In the USA, between 10% and 25% of the ground waters used as a drinking water source has nitrate concentration above the maximum allowable concentration of 10 mg/L NO3--N[2].
Biological denitrification has become very important in modern wastewater and groundwater treatment as a technique of removing excess nitrate. This process is environmentally friendly and may be an economical strategy for the reclamation of nitrate-contaminated water[3].
Denitrification is a microbial process that converts nitrate to dinitrogen gas via several intermediates, including nitrite and nitrous oxide[4], NO3(aq)- → NO2(aq)- → NO(enzyme complex)→ N2O(gas) →N2(gas). The transformations involve the reduction of nitrogen, and so, require a source of electron donors (e.g. acetate, hydrogen gas, elemental sulphur, thiosulphate, aqueous ferrous iron and pyrite).
Recently, autotrophic denitrification processes including sulfur-based denitrification and hydrogen-based autotrophic denitrification[5-6], were widely studied in the remediation of groundwater and drinking water.
Sulfur-based autotrophic denitrification is considered as an alternative process for nitrate removal from drinking water[7]. Meanwhile, sulfur-based autotrophic denitrification involves the use of reduced sulfur (e.g. hydrogen sulfide, elemental sulfur) as the electron donor and nitrate as the terminal electron acceptor in the denitrifying bacteria’s electron transport chain (Eq. (1) )[8].
| $\eqalign{ & 1.0NO_3^ - + 1.1OS + 0.40C{O_2} + 0.76{H_2}O + \cr & 0.080NH_4^ + \to 0.080{C_5}{H_7}{O_2}N + 0.50{N_2} + \cr & 1.10SO_4^{2 - } + 1.28{H^ + } \cr} $ | (1) |
Similar to heterotrophic denitrifying bacteria, sulfur based autotrophic denitrifiers require anoxic conditions, though the carbon source required for this chemo-autotrophic process is derived from inorganic carbon (e.g. carbon dioxide, bicarbonate). Besides, as elemental-sulfur is non-toxic, water insoluble, stable under normal conditions, and readily available, much more attention has been paid to sulfur-based autotrophic denitrification of nitrate-contaminated groundwater[9].
Nitrate-dependent Fe (Ⅱ) oxidation to Fe (Ⅲ) by bacteria has been only recently described. Straub et al. obtained the first Fe (Ⅱ) oxidizing, nitrate reducing (enrichment) culture from brackish water lagoon sediment[10].This culture grew fully autotrophically, using ferrous iron as the only electron donor and nitrate as the electron acceptor, according to the reaction (Eq. (2) )[11]:
| $\eqalign{ & 5F{e^{2 + }} + NO_3^ - + 12{H_2}O = \cr & 5Fe{(OH)_3} + 0.5{N_2} + 9{H^ + } \cr} $ | (2) |
Hydrogen gas is a safe alternative to organic electron donors and element sulphur. It is non-toxic and without unwanted by products[12]. Furthermore, it is cheap and generates 50% less microbial biomass than traditional electron donors such as methanol[13]. By using hydrogen gas as the electron donor, hydrogenotrophic denitrification can occur in the absence of oxygen and nitrate is reduced to nitrogen. However, bicarbonate-carbon to nitrate nitrogen ratio of 2: 1 was added to ensure that nitrate was the limiting nutrient based on the stoichiometry of (Eq. (3) )[14](C: N=0.21: 1)
| $\eqalign{ & {H_2} + 0.33NO_3^ - + 0.08C{O_2} + 0.34{H^ + } \to \cr & 0.015{C_5}{H_7}N{O_2} + 1.11{H_2}O + 0.16{N_2} \cr} $ | (3) |
Generally, denitrification rate is particularly affected by pH, temperature, and C/N ratio. The effects of these factors on autotrophic denitrification rate with different donors have been investigated. For example, it is well established that pH has a marked effect on the efficiency of the denitrification process, and controlling the pH can improve the performance of denitrification processes[15]. Hao et al.[16] optimized the operating parameters (C/N and HRT) in a three dimensional BER. It has been reported that nitrification and denitrification are strongly inhibited at temperatures[17].
As a candidate for anaerobic denitrification, the strain Y12 isolated from reservoir sediments was identified as Acinetobacter. To our best knowledge, no relative study has been performed to focus on the characteristics of different electron donors. In the present study, the effect of temperature, pH, and C/N ratio on nitrate removal with different electron donors (hydrogen gas, Iron (Ⅱ), sodium sulfide) was further tested. In addition, the growth curve of Y12 was also investigated with respect to the autotrophic denitrification at the anaerobic condition.
2 Materials and Methods 2.1 Sampling and Culture Medium PreparationThe sediment samples were collected from Tang Yu reservoir, Shanxi Province, China in March 2014 and used to isolate autotrophic denitrification bacteria.
The ingredients of enrichment medium (pH 6.8-7.0) in 1 000 mL distilled water were as follows: 1.0g of NaHCO3, 0.3 g of NaNO3, 0.1 g of KH2PO4, 0.3 g of FeCl2, 0.3 g of Na2S, 0.05 g of MgSO4·7H2O, 0.05 g of CaCl2 and 2 mL of trace elements solution.The basal medium (pH 6.8) consisted of the following components:0.5 g of NaHCO3, 0.1 g of NaNO3, 0.1 g of KH2PO4, 0.05 g of MgSO4·7H2O, 0.05 g of CaCl2, 0.1 g of FeCl2, 0.1 g of Na2S and 2 mL of trace elements solution. The final pH of the medium was adjusted by 1 mol/L NaOH or HCl solution. The trace elements solution contained 0.5 g of MgSO4·7H2O, 1.0 g of EDTA, 0.2 g of ZnSO4, 0.1 g of MnCl2·4H2O, 0.5 g of FeSO4·7H2O, 0.5 g of CuSO4·5H2O and 0.2 g of CoCl2·6H2O (per liter).
10mL sediment samples were inoculated into 100 mL of enrichment medium, incubated at 30 ℃ for 2 weeks at the anaerobic condition. After enrichment culture, the enrichment suspension (1 mL) was transferred to 100 mL of the basal medium and incubated under the same culture conditions. All enrichment steps were carried out under sterilized conditions. Resultant bacterial suspension was spread on the basal medium plates and incubated at 30 ℃. Separate colonies were picked and purified by repeated streaking on the fresh basal medium plates. Then, the isolates were cultivated in the fresh basal medium to test their ability of denitrification. Finally, the strain Y12 with the highest denitrification ability was selected for further study and stocked in 20% glycerol solution at -20 ℃.
2.2 Strain Identification and Denitrification Gene AmplificationThe 16S rRNA gene of the isolate Y12 was amplified using bacterial universal primers F27 (5′-AGAGTTTGATCMTGGCTCAG-3′) and R1492 (5′-TTGGYTACCTTGTTACGACT-3′). The PCR was carried out as follows: 5 min at 94 ℃, 30 cycles of 1 min at 94 ℃, 1 min at 53 ℃, 1.5min at 72 ℃ and a final step of 10 min at 72 ℃. PCR products were run and visualized on a 1% agarose gel electrophoresis and ethidium bromide staining. The amplified products were purified and sequenced by TaKaRa Biotechnology Co. Ltd (Dalian, China). Finally, sequences were compared to other relevant microorganisms in GenBank by BLAST and submitted for their accession numbers.
2.3 Effect of Different Electron Donors on Nitrate RemovalTo estimate the nitrate removal capability of the strain Y12, the experiment was performed by using FeCl2, H2, Na2S as electron donors, respectively. The 250 mL bottles with 200 mL sterile basal medium were incubated at 30 ℃ and 10% of inoculum by volume was added to the bottles. However, the rates of nitrate removal were noticeably different depending on the electron donors supplied.
So, the bottles were performed for a period of 192hours. All microcosms were prepared and incubated in anaerobic chamber. The different electron donors (hydrogen, sodium sulfide, ferrous iron) were added in the bottles with different ways. Hydrogen was added by first evacuating the microcosm headspace, then delivering hydrogen into the bottle by syringe. Hydrogen was sparged once per day, in the evenings while feedings were carried out in the mornings. Stock solutions of sodium sulfide and anaerobically prepared ferrous iron solution were filter-sterilized. After inoculation, the solutions were added into the bottles with sterile basal medium. Meanwhile, temperature controller was adjusted to control temperature. The bottles were monitored every other day and aliquots of 10 mL were removed periodically to determine nitrate and OD600.
2.4 Analytical MethodsSamples were filtered using cellulose acetate syringe filters with pore size of 0.45nm before the measurements of nitrate. The optical density of the isolate was measured at OD600 nm using an ultraviolet spectrophotometer (DR5000, HACH, USA). Nitrate-N and pH were determined according to standard methods[18]. Briefly, the nitrate concentration was determined following a UV spectrophotometric method to differentiate the difference between OD220 and 2×OD275.
2.5 Statistical AnalysisNitrate removal ratio formula was (ρ0-ρn)/ρ0×100%. ρ0 is the initial concentration of NO3--N and ρn is the final concentration of NO3--N at n hour. Data in this experiment was analyzed by Microsoft Excel and Origin 9.0 software.
3 Results and Discussion 3.1 16S rRNA Gene Sequencing and Similarity AnalysisAn autotrophic denitrification bacterium Y12 was isolated from Tang Yu reservoir. The strain Y12 had the ability to repeatedly use inorganic carbon (e.g. sodium bicarbonate) as a carbon source with H2, Na2S, Fe (Ⅱ) as the electron donors and nitrate as the electron acceptor. The BLAST results indicated that strain Y12 was closely related to members of genus Acinetobacter, showing the highest similarity (99%) to Acinetobacter sp. (NR026207) . A phylogenetic tree was constructed based on 16S rRNA gene sequence of the isolate and some other phylogenetically related strains (Fig. 1). Therefore, Y12 was identified to be an Acinetobacter species.
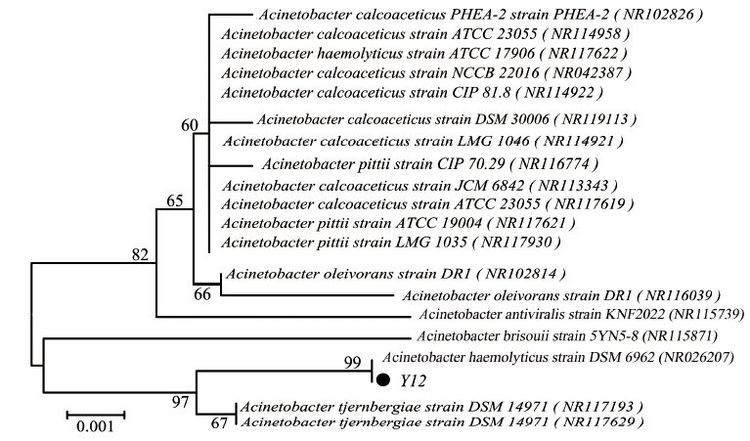
|
The tree was constructed using neighbor joining method with Bootstrap values Figure 1 Phylogenetic tree based on the comparison of partial 16S rRNA gene sequences of strain Y12 |
3.2 Cell Growth Characteristics
To characterize the growth of the pure isolate, the pure culture was enriched in anaerobic bottles at 30 ℃ about 5 days. When the culture had become obviously turbid, 25 mL of cell suspension was transferred into 250 mL bottles with 200 mL sterile basal medium. The results showed the Y12 grew well and could utilize different electron donors (hydrogen gas, sodium sulfide, ferrous iron) at 30 ℃ and pH 7.0. The growth profiles of the isolate strain Y12 was plotted in Fig. 2.
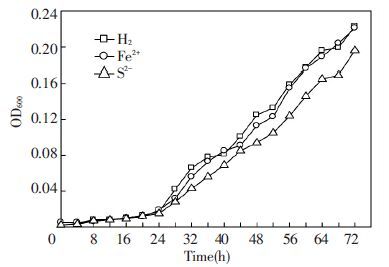
|
Figure 2 Cell growth characteristics of Y12 in the basal medium at 30 ℃ at the anaerobic condition |
The ability of the Y12 to utilize hydrogen, sodium sulfide and ferrous iron as electron donors for growth was investigated. We observed a consistently higher growth rate in all cases when used Fe2+ and H2 as electron donors than used S2- as electron donor. Indicating that autotrophic denitrification could not only occur in anaerobic condition, but also utilize nitrate with different electron donors (hydrogen gas, sodium sulfide, ferrous iron). Under autotrophic growth conditions, carbon dioxide or bicarbonate was used as carbon source for microbial cell synthesis. As a result, in the presence of hydrogen gas, sodium sulfide and ferrous iron, cells grew exponentially with a doubling time of 24 h. Moreover, maximum growth was observed between 24 and 64 h, and the stationary phase began after 64 h of cultivation. Furthermore, in batch culture was carried out under the autotrophic conditions and strain AHO1 always started to grow after a lag phase of 1-3 days [19].
3.3 Nitrate Reduction in the Presence of Different C/N RatioSuitable amounts of carbon and nitrogen source should be supplied to cope with the susceptibility of inhibition or acceleration during the denitrification process. Therefore, carbon to nitrogen ratios (C/N) must be maintained at appropriate levels in autotrophic denitrification process. Many researchers had investigated the effects of different parameters and tried to optimize C/N. For example, Hao et al.[16] optimized the operating parameters (C/N and HRT) in a three-dimensional BER. The C/N was tested at 0.5, 1.0, 1.5, 2.0, 3.0, and the HRT at 5, 7, 10, 12 h. A nitrate removal efficiency of 98.3% was obtained at C/N ratio of 3.0 and HRT of 7 h. According to Wang et al.[20], the C/N ratio of 1.00 had not been sufficient for the bacteria.
Therefore, the C/N ratios of basal medium were adjusted to 1.5, 3.0, 4.5, 6.0, and 9.0, respectively. The amount of sodium bicarbonate was changed to adjust the C/N ratios in the basal medium by maintaining a constant nitrate nitrogen concentration at 100 mg/L.
When the initial C/N ratio of the medium was 1.5, 3.0, 4.5, 6.0 and 9.0, respectively, after 192 h cultivation at pH=7 and t=30 ℃, as shown in Fig. 3. The bacterium Y12 was chemolithoautotroph and could use inorganic material (such as hydrogen gas, ferrous iron or sulfide) as an electron donor. The experiments were carried out at the anaerobic conditions. When used ferrous iron as electron donor, C/N ratios had little effect on autotrophic denitrification. The nitrate removal ratio of 100.00% could be obtained at an initial C/N ratio from 1.5 to 9.0. And, all nitrate nitrogen could be completely removed.
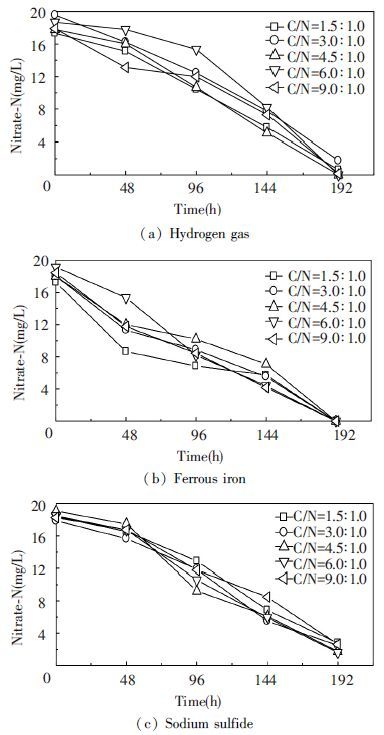
|
Figure 3 Comparison of nitrate nitrogen concentration in the presence of various C/N ratios |
When used hydrogen gas and sodium sulfide as electron donors, a maximum nitrate removal ratio of 100.00% and 91.43% could be obtained at the initial C/N ratio of 6.0. At the same time, the nitrate degradation ratio gradually increased with the increasing C/N ratio from 1.5 to 6.0, and a further increase in C/N (above 6.0) did not result in further improvement of nitrate degradation ratio. And, the nitrate degradation ratio increased with the increasing C/N ratio from 4.5 to 6.0 was over 90%. As a result, the C/N ratio of 4.5 was sufficient for the growth of denitrifying bacteria if only bicarbonate was added as carbon source. Analysis from the economic perspective, C/N ratio of 4.5 was more suitable and desirable for denitrification. In previous research, a similar reported C/N ratio was also observed. The C to N ratio of 3.75: 1 was optimal for complete denitrification. At the optimal ratio, no residual NO3--N was detected after 5 days[21].It was reported that the denitrification was performed primarily using hydrogen gas as an electron donor when the C/N ratio less than the stoichiometric ratio[22].
The denitrification rate of Y12 with ferrous iron, hydrogen gas and sodium sulfide as electron donors was affected by C/N ratios. It was presumably because the absorption atmospheric CO2, CO2 was dissolved in water and CO32- was generated according to Eqs. (4) and (5) [23].
| $C{O_2} + {H_2}O \to HCO_3^ - + {H^ + }$ | (4) |
| $HCO_3^ - + O{H^ - } \to CO_3^{2 - } + {H_2}O$ | (5) |
Water temperature affects different biological denitrification processes. For example, denitrification rates at 14 ℃ were approximately one third of the rates at 32 ℃ when newspaper was used as a carbon source for heterotrophic denitrification[24]. In this study, when the initial temperature of the medium was 15, 20, 25, 30 and 35 ℃, respectively. After cultivation of 192 h at C/N=5.0: 1 and pH=7, Fig. 4 illustrates profiles for the nitrate removal during the experimental period.When used hydrogen gas, ferrous iron and sodium sulfide as electron donors, a maximum nitrate removal ratio of 100.00%, 91.43% and 87.99% could be obtained at the temperature of 30 ℃, respectively.And, the fastest nitrate removal ratios were observed with ferrous iron and hydrogen gas as electron donors. At the same time, the nitrate degradation ratio gradually increased with the temperature from 15 ℃ to 30 ℃. Then, the degradation ratio of nitrate dropped slightly with a further increase in temperature (above 30 ℃). The optimal temperature for strain Y12 was 30 ℃, which could be verified from the highest nitrate removal ratios and the fastest growth rate under this temperature.
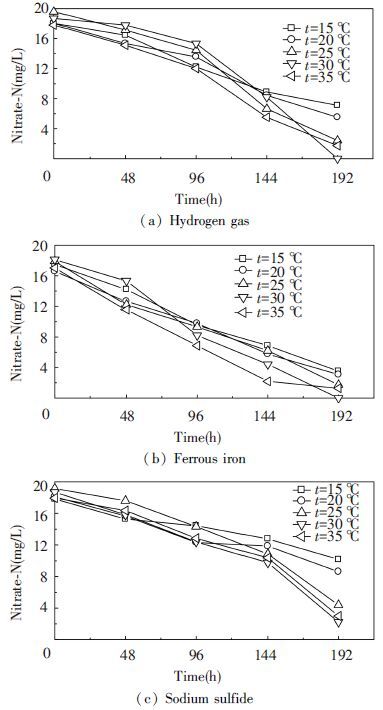
|
Figure 4 Comparison of nitrate-N concentration in the presence of various temperature |
Temperature is an external factor that is well known to play a significant role in bacterial growth. The optimum temperature for denitrification was between 25 and 35 ℃[25]. Where, bacteria had evolved to cope with specific environmental conditions. Therefore, the results obtained were consistent with the reported in the literature. Experimental evidence suggested that the temperature affects the denitrification process by affecting bacteria behavior. High temperatures favored autotrophic denitrification process as the batch test. Reduction of 10 mg/L of nitrate was accomplished within 150 min at 28 ℃, but nitrate concentration was only finished half in 250 min at 8 ℃[26].
3.5 Nitrate Reduction in the Presence of Different pHThe rate of autotrophic denitrification by reaction with different donors is also pH controlled. Strongly acidic environments (pH<5) inhibited denitrification and tended to arrest the denitrification chain with the formation of nitrite or N2O[25].
As shown in Fig. 5, when the initial pH of the medium was 5.0, 6.0, 7.0, 8.0 and 9.0, respectively, after 192 h cultivation at C/N=5.0: 1 and t=30. Maximum denitrification activity was observed at pH 6.0-7.0. Thus, when used hydrogen gas and sodium sulfide as electron donors, a maximum nitrate removal ratio of 100.00% and 87.39% at the pH of 6.0. However, when used ferrous iron as electron donor, a maximum nitrate removal ratio of 100.00% was observed at pH 7.0. At the same time, the nitrate degradation ratio gradually increased with the increasing pH from 5.0 to 7.0. Then, the degradation ratio of nitrate dropped sharply with a further increase in pH (above 7.0) . The reason may be that internal environment of the medium was no longer suitable for autotrophic denitrifying bacteria. The results showed that the optimal range of pH for autotrophic denitrifier Y12 was 6.0-7.0.
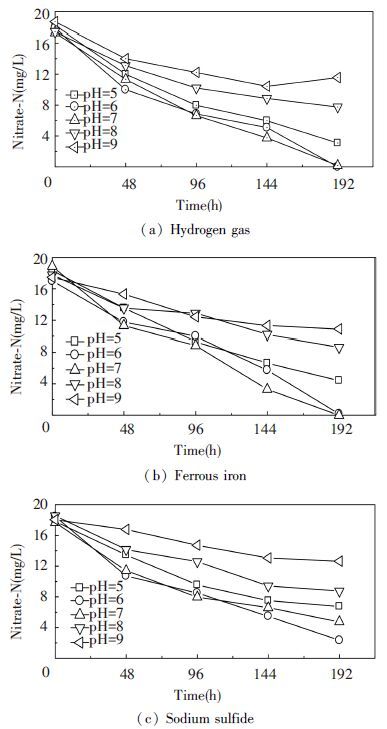
|
Figure 5 Comparison of nitrate-N concentration in the presence of various pH |
The effects of pH were comparable to those reported by other researchers using autotrophic denitrifiers and nitrate as nitrogen source. Vidal et al.[27] observed that the optimal range of pH for autotrophic denitrifiers was 6.8-8.2. The optimal denitrification performance could be obtained under conditions of pH 6.8-7.0[28]. So, the strain Y12 had a good denitrification capability in neutral or acidic condition. These researches were very similar to this study.
4 ConclusionIn this study, a strain Y12 was isolated from the sediment of Tang Yu reservoir, and identified as the genera of Acinetobacter sp. When used ferrous iron as electron donor, the nitrate could be completely removed at the initial C/N ratio from 1.5to 9.0. Furthermore, when used hydrogen gas and sodium sulfide as electron donors, a maximum nitrate removal ratio of 100.00% and 91.43% were obtained at the C/N ratio of 6.0. The optimal temperature for strain Y12 was 30 ℃. When made use of hydrogen gas, ferrous iron and sodium sulfide as electron donors, a maximum nitrate removal ratio of 100.00%, 91.43% and 87.99% at the temperature of 30 ℃, respectively. Maximum denitritation activity was observed at pH 6.0-7.0.
| [1] |
Sahinkaya E, Kilic A. Heterotrophic and elemental sulfur based autotrophic denitrification processes for simultaneous nitrate and Cr (VI) reduction.
Water Research,2014, 50 : 278-286.
( 0) 0)
|
| [2] |
Liu H, Jiang W, Wan D, et al. Study of a combined heterotrophic and sulfur autotrophic denitrification technology for removal of nitrate in water.
Journal of Hazardous Materials,2009, 169 (1/2/3) : 23-28.
( 0) 0)
|
| [3] |
Soares M I M. Biological denitrification of groundwater.
Water, Air, Soil Pollut,2000, 123 (1/2/3/4) : 183-193.
( 0) 0)
|
| [4] |
Page A L.
Methods of soil analysis: part 2. chemical and microbiological properties. 2nd ed. Madison: WI: 1982 : 1011 -1026.
( 0) 0)
|
| [5] |
Koenig A, Zhang T, Liu L H, et al. Microbial community and biochemistry process in autosulfurotrophic denitrifying biofilm.
Chemosphere,2005, 58 (8) : 1041-1047.
( 0) 0)
|
| [6] |
Lee K C, Rittmann B E. A novel hollow fiber membrane biofilm reactor for autohydrogenotrophic denitrification of drinking water.
Water Sci Technol,2000, 41 : 219-226.
( 0) 0)
|
| [7] |
Soares M I M. Denitrification of groundwater with elemental sulfur.
Water Res,2002, 36 (5) : 1392-1395.
( 0) 0)
|
| [8] |
Pan S H.
Autotrophic denitrification of groundwater in a granular sulfur-packed up-flow reactor. Texas: The University of Texas at Arlington, 2007 .
( 0) 0)
|
| [9] |
Moon H S, Shin D Y, Nam K, et al. A long-term performance test on an autotrophic denitrification column for application as a permeable reactive barrier.
Chemosphere,2002, 73 (5) : 723-728.
( 0) 0)
|
| [10] |
Straub K L, Benz M, Schink B, et al. Anaerobic, nitratedependent microbial oxidation of ferrous iron.
Appl Environ Microbiol,1996, 62 (4) : 1458-1460.
( 0) 0)
|
| [11] |
Blöthe M, Roden E E. Composition and activity of an autotrophic Fe(Ⅱ)-oxidizing, nitrate-reducing enrichment culture.
Appl Environ Microbiol,2009, 75 (21) : 6937-6940.
( 0) 0)
|
| [12] |
Mansell B O, Schroeder E D. Hydrogenotrophic denitrification in a microporous membrane bioreactor.
Water Res,2002, 36 (19) : 4683-4690.
( 0) 0)
|
| [13] |
Ergas S J, Reuss A F. Hydrogenotrophic denitrification of drinking water using a hollow fiber membrane bioreactor.
J Water Supply Res Technol-AQUA,2001, 50 (3) : 161-217.
( 0) 0)
|
| [14] |
McCarty P L.
Stoichiometry of biological reactions. Atlanta: International Conference Towards a Unified Concept of Biological Waste Treatment Design, 1972 .
( 0) 0)
|
| [15] |
Rezania B, Cicek N, Oleszkiewicz J A. Kinetics of hydrogen-dependent denitrification under varying pH and temperature conditions.
Biotechnol Bioeng,2005, 92 (7) : 900-906.
( 0) 0)
|
| [16] |
Hao R, Li S, Li J, et al. Denitrification of simulated municipal wastewater treatment plant effluent using a three-dimensional biofilm electrode reactor: operating performance and bacterial community.
Bioresour Technol,2013, 143 : 178-186.
( 0) 0)
|
| [17] |
Carrera J, Vicent T, Lafuente F J. Influence of temperature on denitrification of an industrial high-strength nitrogen wastewater in a two-sludge system.
Water S A,2003, 29 : 11-16.
( 0) 0)
|
| [18] |
AP HA.
Standard Methods for the Examination of Water and Wastewater.19th ed. American Public Health Association: Washington D.C, 1995 .
( 0) 0)
|
| [19] |
Yu D, Sorokin T P, TourovaJ G, et al. A new facultatively autotrophic hydrogen and sulfur oxidizing bacterium from an alkaline environment.
Extremophiles,2000, 4 (4) : 237-245.
( 0) 0)
|
| [20] |
Wang Q H, Feng C P, Zhao Y X, et al. Denitrification of nitrate contaminated groundwater with a fiber-based biofilm reactor.
Bioresour Technol,2009, 100 (7) : 2223-2227.
( 0) 0)
|
| [21] |
Huang G X, Fallowfield H, Guan H D, et al. Remediation of nitrate nitrogen contaminated groundwater by a heterotrophic-autotrophic denitrification.
Approach in an Aerobic Environment,2012, 223 (7) : 4029-4038.
( 0) 0)
|
| [22] |
Zhou M, Fu W, Gu H, et al. Nitrate removal from groundwater by a novel three-dimensional electrode biofilm reactor.
Electrochimica Acta,2007, 52 (19) : 6052-6059.
( 0) 0)
|
| [23] |
Zhao Y X, Feng C P, Wang Q H, et al. Nitrate removal from groundwater by cooperating heterotrophic with autotrophic denitrification in a biofilm-electrode reactor.
Journal of Hazardous Materials,2011, 192 (3) : 1033-1039.
( 0) 0)
|
| [24] |
Volokita M, Abeliovich A, Soares M I M. Denitrification of groundwater using cotton as energy source.
Water Science and Technology,1996, 34 (12) : 379-385.
( 0) 0)
|
| [25] |
Brady N C, Weil R R.
The Nature and Properties of Soils.13th ed. New Jersey: Prentice Hall, 2001 .
( 0) 0)
|
| [26] |
Zhou W L, Sunl Y, Wu B T, et al. Autotrophic denitrification for nitrate and nitrite removal using sulfur-1imestone.
Journal of Environmental Sciences,2011, 23 (11) : 1761-1769.
( 0) 0)
|
| [27] |
Vidal S, Rocha C, Galvao H. A comparison of organic and inorganic carbon control over biological denitrification in aqua.
Chemosphere,2002, 48 (4) : 445-451.
( 0) 0)
|
| [28] |
Wang H Y, Yang K, Zhang Q, et al. Nitrate removal by a strain of nitrate-dependent Fe(Ⅱ) oxidation bacteria.
Environmetal Science,2014, 35 (4) : 1437-1442.
( 0) 0)
|
 2016, Vol. 23
2016, Vol. 23


