2. Shandong Provincial Engineering Technology Research Center of Marine Health Food, Rongcheng 264300, Shandong, China;
3. National and Local United Engineering Laboratory of Marine Functional Food Development (Shandong), Rongcheng 264300, Shandong, China
Spontaneously, free radicals and antioxidant substances play significant roles in flora and fauna[1]. Oxidative injuries have been observed to be associated with the pathogenesis of many chronic diseases[2]. Reactive oxygen species (ROS) such as superoxide anion radical (O2-·), hydroxyl radical (·OH) and hydrogen peroxide (H2O2) are normally produced in living organisms during metabolism. Antioxidants can scavenge ROS and prevent the cell injuries, thereby playing an important role in human health[3].
At present, synthetic chemicals, such as Butylated hydroxyanisole (BHA), Butylated hydroxytoluene (BHT), Propyl gallate (PG) and Tert-butylhydroquinone (TBHQ) are used extensively in the inhibition of the oxidation processes in foods and cosmetics, and showed potential risks to human health[4-6]. Therefore, it was of vital importance to do researches on the safe and nutrient natural antioxidant substances derived from the food ingredient, during the last few decades[7].
Over the years, those bioactive peptides with antioxidant activity were usually obtained from various food sources' protein hydro-lysates, such as dairy, meat, fish, soy and egg, with various enzymes[8]. The increased attentions have been paid to the purification and applications of bioactive peptides from natural biomass sources, especially the low molecular marine protein hydro-lysates[9]. Furthermore, many studies reported that antioxidant peptides could be applied to protect human health due to their excellent antioxidant capabilities[10-11].
Marine scallop, especially Mimachlamys nobilis (M. nobilis), a kind of abundant, tasty and nutrition-rich marine bivalve at both a commercial level and an ecological level, is widely distributed among the coasts of China, Korea, Japan and Indonesia[12]. The M. nobilis is usually used for the processing of scallop meats, which may produce a large amount of by-products (scallop skirts) and pay for the treatment of the waste. Most of the wasted scallop skirts are dumped into landfill, which also cause a lot of pollution. However, the scallop skirts are also rich in nutrients, such as proteins, carbohydrate, vitamins and a variety of microelements. Therefore, to recover the bioactive components from scallop by-products for further utilization, the enzymatic hydrolysis of scallop by-products is the most efficient technology to recover added-value peptides with functional, biological and nutritional properties[13-14]. The objectives of this study were to determine the appropriate protease for preparing the scallop skirts protein hydro-lysates and further to purify and characterize the antioxidant peptides.
2 Materials and Methods 2.1 MaterialsFresh scallop (Mimachlamys nobilis) was bought from a local market in Weihai, China. Various proteases such as papain (18 000 U/g), trypsin (20 000 U/g), pepsin (20 000 U/g) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), and alkaline protease (40 000 U/g), neutrase (15 000 U/g) were bought from Beijing Aoboxing Bio-tech Co., Ltd. (Beijing, China). 1, 1-diphenyl-2-pycryl-hydrazyl (DPPH), Sephadex G-25 and G-15 were purchased from Sigma Chemical Co. (St. Louis, MO). All other chemicals used were of analytical grade.
2.2 Preparation of Scallop Skirts Hydro-lysatesIn order to prepare the hydro-lysates of the scallop by-products, enzymatic hydrolysis was performed using various enzymes (papain, trypsin, pepsin, alkaline protease and neutrase) at their optimal conditions. The boiled scallop skirts were separately hydrolyzed with various enzymes for 5 h, and the additive amount of every enzyme was 1 200 U per gram substrate, under the optimum pH and temperature conditions (Table 1). After the reactions, the systems were boiled at 100 ℃ for 15 min to terminate the enzyme activity. Each hydro-lysate was centrifuged at 4 500 ×g for 15 min, and the supernatant was collected for the activity analysis.
| Table 1 Optimum conditions of enzymatic hydrolysis for various enzymes |
2.3 Purification of Antioxidative Peptides
The supernatants of the hydro-lysates were fractioned through the ultrafiltration membranes with the 3 kDa and 10 kDa cut-off, then centrifuged at 4 500 ×g for 15 min, the fraction with the highest radical scavenging activity (RSA) was collected at 4 ℃.
The freeze-dried highest RSA fraction was loaded onto a Sephadex G-25 gel filtration column (1×50 cm) which was equilibrated with distilled water, then the column was eluted with distilled water at a flow rate of 0.5 mL/min using nucleic acid protein detector while the addition of the fraction was 1.5 mL. The antioxidant fractions were collected and stored at 4 ℃. Then the freeze-dried fraction showed the highest RSA was loaded onto a Sephadex G-15 gel filtration column (1×50 cm), the elution requirements were just the same as G-25 gel filtration. The eluent with peak at 280 nm was collect and stored at 4 ℃, and the RSA was measured.
After that, the highest RSA component was freeze-dried and then separated by reversed-phase high performance liquid chromatography (RP-HPLC) on the Agilent C18 column (5 μm, 4.6 × 250 mm) using acetonitrile (5%, volume ratio) containing 0.1% trifluoroacetic acid (TFA) at a low rate of 0.4 mL/min and measured at 220 nm.
2.4 Analysis of SDS-PAGEAccording to the consequence of the RP-HPLC, the freeze-dried final antioxidative peptides component was collected and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli with slight modifications[15]. The purified peptides, along with a peptide standard (molecular weight 3.3-20.1 kDa) were heated at 100 ℃ for 5 min in SDS-PAGE loading buffer (12% SDS, 6% β-mercaptoethanol, 30% glycerol, 0.1% bromophenol blue and 150 mmol/L Tris-HCl at pH 7.0), and centrifuged at 5 500×g for 5 min. The peptides and marker were loaded into the sample wells of 3% stacking gel and 17% separating gel. The voltage was kept constant at 80 V until the tracking dye was close to the bottom of the gel. After that, the gel was stained using Coomassie Blue R-250 (0.1%) and destained with double distilled water.
2.5 Identification of Peptides by MALDI-TOF-MSAfter the RP-HPLC purification, the accurate molecular mass of the purified peptides were subjected to Matrix-Assisted Laser Desorption/Ionization Time-of-flight tandem mass spectroscopy (MALDI-TOF-MS) using AXIMA-CFR Plus MALDI-TOF system (KRATOS Analytical, Shimadzu corporation, Japan) at the Institute of Biophysics, Chinese Academy of Sciences (Beijing, China).
2.6 Determination of Antioxidant Activities 2.6.1 DPPH radical scavenging activity assayDPPH RSA of the hydro-lysates was determined by using the method of Yen and Hsieh[16], with slight modifications. DPPH solution (0.2 mmol/L) dissolved with ethanol was added to the sample solution with equal volume. The mixture was then shaken and stood in the dark at room temperature for 30 min. The absorbance of the mixture was measured at 517 nm by using the UV (ultra violet) spectrophotometry. For the blank, equal volume of ethanol was used instead of the sample. DPPH RSA was calculated as follows:
| $ {\rm{DPPH}}\;{\rm{RSA}}\left( \% \right) = \left( {{A_{{\rm{blank}}}} - {A_{{\rm{sample}}}}} \right)/{A_{{\rm{blank}}}} \times 100 $ | (1) |
where Ablank is the absorbance of blank and Asample is the absorbance of the sample.
2.6.2 Hydroxyl radical scavenging activity assayThe hydroxyl RSA of hydro-lysates or peptides was determined according to the method of Wang with some modifications[17]. 1 mL of 6 mmol/L FeSO4 solution was mixed with 1 mL of sample solution and 1 mL of 6 mmol/L H2O2 solution. The mixture was shaken and left for 10 min at room temperature, and then 1 mL of 6 mmol/L salicylic acid was added to the mixture. After 30 min, the absorbance of the mixture was determined at 510 nm. For the blank, 1 mL of distilled water was used instead of the sample. The hydroxyl RSA was calculated as follows:
| $ {\rm{Hydroxyl}}\;{\rm{RSA}}\left( \% \right) = \left[ {1 - \left( {{A_{{\rm{sample}}}} - {A_0}} \right)/{A_{{\rm{blank}}}}} \right] \times 100 $ | (2) |
where Ablank is the absorbance of the blank, Asample is the absorbance of the sample and A0 is the absorbance of the sample without salicylic.
2.6.3 Superoxide anion radical scavenging activity assaySuperoxide anion radicals were generated by the modified pyrogallol autoxidation method of Marklund and Marklund[18]. Briefly, 1 mL of hydro-lysates or peptides was mixed with 3 mL of 50 mmol/L Tris-HCl buffer (pH 8.2) containing 1 mmol/L EDTA and pyrogallic acid (0.5 mL, 4.5 mmol/L). Then the mixture was shaken rapidly at room temperature. The absorbance of the mixture was measured at 320 nm every 30 s for 4 min, and a slope was calculated as the absorbance/min. For the blank, 1 mL of distilled water was used instead of the sample. The superoxide radical anion scavenging activity was calculated as follows:
| $ {\rm{Superoxide}}\;{\rm{anion}}\;{\rm{RSA}}\left( \% \right) = \left( {{\rm{1}} - {A_{{\rm{sample}}}}/{A_{{\rm{blank}}}}} \right) \times 100 $ | (3) |
where Ablank is the change speed of absorbance of the blank group in the superoxide radical anion generation system and Asample is the change speed of absorbance of the sample.
2.7 Statistic AnalysisAll the tests were repeated in triplicated. Data were evaluated using SPSS 19.0 software. Values were expressed as the mean ± standard deviation (SD). The mean values were analyzed using the one-way analysis of variance (ANOVA) test. he significant difference was determined with a 95% confidence interval (p < 0.05).
3 Results and Discussion 3.1 Preparation of Protein Hydro-lysates and Their Antioxidant PropertiesThe scallop (M. nobilis) was concentrated on a 55 μm mesh filter, washed with filtrated seawater, and briefly rinsed in distilled water. For antioxidant peptides productions, the boiled scallop skirts were separately hydrolyzed by various enzymes at their optimal conditions. The RSA of different hydro-lysates was measured using DPPH, hydroxyl and superoxide anion RSA assays. All of the hydro-lysates showed the abilities of scavenging radicals, and the peptides from trypsin hydrolysis indicated the highest ability (75.6%, 79.5% and 76.7% using DPPH, hydroxyl and superoxide anion RSA assays) (Fig. 1). Nevertheless, RSA of all hydro-lysates were lower than those of BHA and TBHQ, which were 87.5% and 90.2%, respectively[19]. Due to the results, the fraction hydrolyzed by trypsin was selected for the further study.
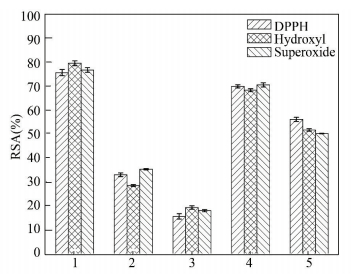
|
Notes : 1. Trypsin; 2. Neutrase; 3. Pepsin; 4. Alkaline protease; 5. Papain Figure 1 Radical scavenging activity (RSA) of Mimachlamys nobilis scallop skirts hydro-lysates prepared by various enzymes |
Antioxidant peptides were obtained by enzymatic modifications of various proteins derived from natural bio-resources. It was reported that in the study of the antioxidant peptides isolated from Ostrich (Struthio camelus) egg white protein, similar to this study, the fraction hydrolyzed by trypsin showed highest RSA among all the enzymes (trypsin, α-chymotrypsin, papain and pepsin)[4]. It was reported that corn gluten meal was separately hydrolyzed by three different enzymes (alkaline protease, trypsin and papain) and peptic hydro-lysates exerted the highest antioxidant activity[20]. Besides, the tuna back bone protein was hydrolyzed by six enzymes (alcalase, α-chymotrypsin, neutrase, papain, pepsin and trypsin) for production of antioxidative peptides and papain hydro-lysates exerted the highest antioxidant activity[21]. It was also reported that the antioxidative peptides were isolated and identified from porcine collagen hydro-lysate with protease bovine pancreas[22]. In addition, the antioxidant peptides purified from duck egg white protein hydro-lysates were hydrolyzed using alcalase[23]. Most of the proteases can be used in the enzymatic hydrolysis of different sources for antioxidant peptides.
3.2 Purification of Antioxidative PeptidesCompared to the large polypeptide, the low molecular weight peptides (2-20 amino acids) are more biologically active[24-25]. Because the low molecular bioactive peptides were essential to metabolic regulation, different ways were used to get peptides with lower molecular mass[26-27]. Ultrafiltration is an effective purification method to obtain low molecular weight peptides from the crude hydro-lysates[28]. Using the ultrafiltration membranes with the 3 kDa and 10 kDa cut-off, the trypsin hydrolysis was divided into three groups due to the molecular weight. The radial scavenging activities was detected by DPPH, hydroxyl and superoxide anion RSA assays, and F2(3-10 kDa) indicated the maximum of RSA, which was more than 77.5%, close to that of unprocessed hydro-lysates (Fig. 2). The RSA of F1 ( > 10 kDa) and F3 ( < 3 kDa) was about 30.0%, which was lower than that of F2. Therefore, F2 was collected for further study.
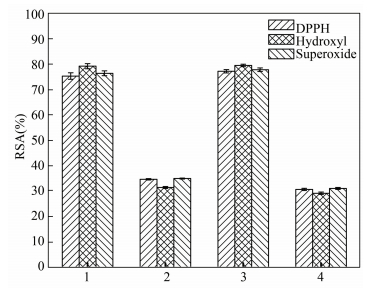
|
Notes: 1. Unprocessed hydro-lysates; 2. F1 > 10 kDa; 3. F2 3-10 kDa; 4. F3 < 3 kDa Figure 2 Radical scavenging activity (RSA) of hydro-lysates of the scallop skirts and the peptides fractions obtained by ultrafiltration (UF) |
In order to purify the antioxidant peptides further, F2 was separated by gel filtration chromatography. It was fractioned according to molecular size using chromatography. Firstly, F2 was loaded onto a Sephadex G-25 gel filtration column and divided into seven fractions, named F2-1 to F2-7. Each fraction was collected and their antioxidant activities were determined (Fig. 3). All of the fractions exhibited the RSA, and F2-3 revealed the highest DPPH RSA, which was 79.6%. Then F2-3 was loaded onto the Sephadex G-15 gel filtration column and four peptide fractions were isolated, named F2-3-1 to F2-3-4, among which, fraction F2-3-2 was identified to be highly component (Fig. 4) and was collected for the next step.
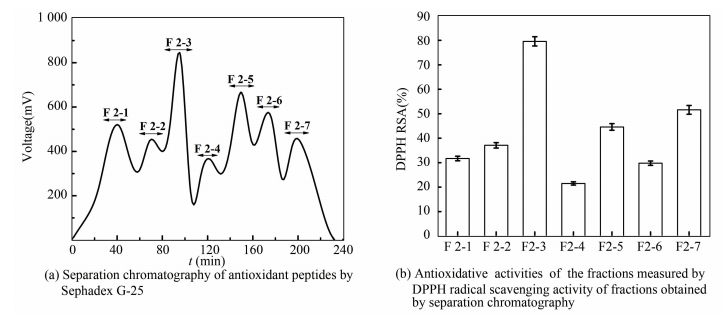
|
Figure 3 Purification of the antioxidant peptide using Sephadex G-25 |
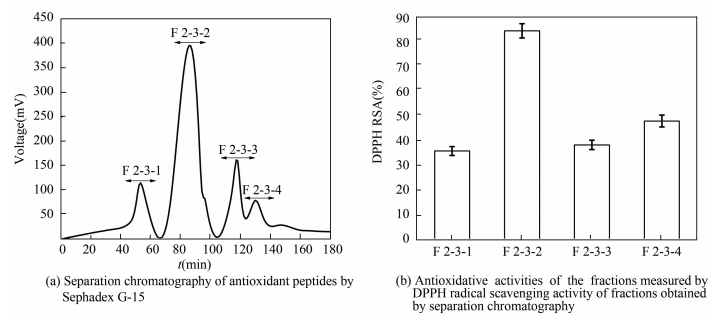
|
Figure 4 Purification of the antioxidant peptide using Sephadex G-15 |
Following the activity analyses, the most potent peptides F2-3-2 was purified with RP-HPLC on an Agilent column (5 μm, 4.6 × 250 mm). One clear peptides peak was identified and collected (Fig. 5). The antioxidant capability of the peak was determined to be 85.7% using DPPH RSA assay (data not shown). The activities of peptide LLPF and FLPF purified from corn gluten meal were 65.13% and 68.67%, respectively, which were obviously lower than the antioxidant capability of the purified peptides of the scallop by-products[20]. Moreover, the antioxidant peptide PHH-Ⅳ isolated from porcine hemoglobin showed lower RSA which was 55.8%[29]. And the peptide purified from croceine croaker (Pseudosciaena crocea) muscle showed the highest RSA, which was 76.74%, lower than the isolated peptides in this study[30]. Among the results of DPPH, hydroxyl and superoxide anion RSA assays, all the target fractions MNAP showed good antioxidant capabilities. The proteins isolated from Mimachlamys nobilis was named MNAP and collected for the further study.
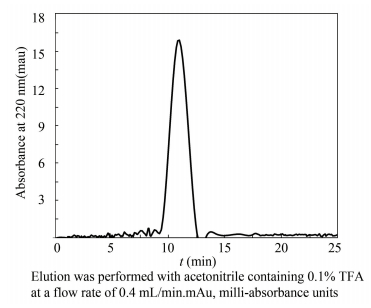
|
Figure 5 RP-HPLC chromatogram for the purification of antioxidant peptides |
3.3 Analysis of SDS-PAGE
The final antioxidant peptides component MNAP was collected and analyzed by SDS-PAGE. As shown in Fig. 6, the SDS-PAGE profile of the antioxidative peptides exhibited a clear peptide band appearing at approximately 3-5 kDa. It was reported that the molecular weights of the antioxidative hydro-lysates isolated from housefly larvae were about 1.6 kDa[17]. And the antioxidant peptides purified from Ficus deltoidea were reported to be approximately 4.4 kDa, higher than those of the peptides in this study[31]. Besides, the antioxidant peptides from Pollachius virens were identified to be 8.0 kDa, which were even higher[19].

|
Figure 6 SDS-PAGE of hydro-lysates MNAP purified by RP-HPLC |
3.4 Proteins Identification by MALDI-TOF-MS
The final antioxidant peptides component MNAP that purified by RP-HPLC was identified by MALDI-TOF-MS with the strong signals. As shown in Fig. 7, two peptides were isolated from the fraction, which were 2 855.6 Da (MNAP1) and 3 007.2 Da (MNAP2), respectively. The result of the molecular weights using MALDI-TOF-MS was close to the analyses of the consequences of ultrafiltration and SDS-PAGE. The molecular weights (Mw) of the antioxidant peptides purified from the marine rotifer (Brachionus rotundiformis) were identified to be 1 076 Da and 1 033 Da, respectively, lower than MNAP1 and MNAP2[19]. It was reported that the molecular weight of the antioxidant peptide isolated from Rana catesbeiana Shaw was 1 988 Da using Quadrupole Time-of-flight mass spectroscopy (Q-TOF-MS), and the molecular mass of the antioxidant peptide isolated from hoki (Johnius belengerii) frame protein was reported to be 1 801 Da using Electrospray Ionization mass spectroscopy (ESI-MS)[7, 32]. Besides, the natural antioxidant peptide separated from fertilized eggs was identified (Mw: 1 291.51 Da) using LTQ-MS[33]. The molecular weights of the peptides purified from the by-products of scallop were close to those reported peptides with antioxidant capabilities. It was also reported that the low molecular peptides were considered to react easily with oxidation radicals and thereby reducing the damage from radicals[34].
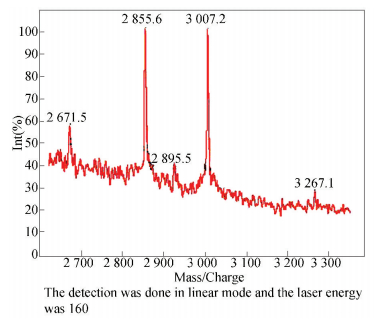
|
Figure 7 MALDI-TOF-MS analysis of RP-HPLC purified antioxidant peptides sample MNAP |
4 Conclusions
Scallop is a very important cash seafood with higher values in food. As the processing wastes of scallop, scallop skirts are rich in nutrients, such as proteins, carbohydrate, vitamins and a variety of microelements. In this study, as the by-products of scallop, scallop skirts protein was recovered using enzymatic hydrolysis with various enzymes and the trypsin hydrolysis exerted the highest antioxidant activity (75.6%, 79.5% and 76.7% using DPPH, hydroxyl and superoxide anion radical scavenging activity assays, respectively). Using UF membranes and GFC processes, antioxidant peptides with potent DPPH, hydroxyl and superoxide anion radical scavenging were purified. Finally, using consecutive chromatographic methods and SDS-PAGE, the antioxidant activity of the isolated peptides MNAP was 85.7% using DPPH RSA assay and their molecular weights were identified, which were 2 855.6 Da (MNAP1) and 3 007.2 Da (MNAP2), respectively. The results suggested that the hydro-lysates from the scallop skirts of Mimachlamys nobilis could be used as natural antioxidants for enhancing antioxidant properties of functional food and for preventing oxidation reaction in food processing.
| [1] |
Matxain J M, Padro D, Ristilä M, et al. Evidence of high *OH radical quenching efficiency by vitamin B6. Journal of Physical Chemistry B, 2009, 113(29): 9629-9632. DOI:10.1021/jp903023c ( 0) 0)
|
| [2] |
Poli G, Biasi F, Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biology, 2013, 1(1): 125-130. DOI:10.1016/j.redox.2012.12.001 ( 0) 0)
|
| [3] |
Sheih I C, Wu T K, Fang T J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresource Technology, 2009, 100(13): 3419-3425. DOI:10.1016/j.biortech.2009.02.014 ( 0) 0)
|
| [4] |
Tanzadehpanah H, Asoodeh A, Chamani J, et al. An antioxidant peptide derived from Ostrich (Struthio camelus) egg white protein hydrolysates. Food Research International, 2012, 49(1): 105-111. DOI:10.1016/j.foodres.2012.08.022 ( 0) 0)
|
| [5] |
Qin L, Zhu B W, Zhou D Y, et al. Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT-Food Science and Technology, 2011, 44(4): 1113-1118. DOI:10.1016/j.lwt.2010.10.013 ( 0) 0)
|
| [6] |
Gülçin İ. Antioxidant activity of food constituents: An overview. Archives of Toxicology, 2011, 86(3): 345-391. ( 0) 0)
|
| [7] |
Kim S Y, Je J Y, Sk K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. Journal of Nutritional Biochemistry, 2007, 18(1): 31-38. DOI:10.1016/j.jnutbio.2006.02.006 ( 0) 0)
|
| [8] |
Dávalos A, Miguel M, Bartolomé B, et al. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. Journal of Food Protection, 2004, 67(9): 1939-1944. DOI:10.4315/0362-028X-67.9.1939 ( 0) 0)
|
| [9] |
Chalamaiah M, Kumar B D, Hemalatha R, et al. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chemistry, 2012, 135(4): 3020-3038. DOI:10.1016/j.foodchem.2012.06.100 ( 0) 0)
|
| [10] |
Chen C, Chi Y J, Zhao M Y, et al. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids, 2012, 43(1): 457-466. DOI:10.1007/s00726-011-1102-0 ( 0) 0)
|
| [11] |
Yang R, Zou Y, Yu N, et al. Accumulation and identification of angiotensin-converting enzyme inhibitory peptides from wheat germ. Journal of Agricultural & Food Chemistry, 2011, 59(8): 3598-3605. ( 0) 0)
|
| [12] |
Qiu L M, Song L S W, Ni D J, et al. Molecular cloning and expression of a toll receptor gene homologue from Zhikong Scallop, Chlamys farreri. Fish & Shellfish Immunology, 2007, 22(5): 451-466. ( 0) 0)
|
| [13] |
Sila A, Sayari N, Balti R, et al. Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chemistry, 2014, 148(4): 445-452. ( 0) 0)
|
| [14] |
Shahidi F, Han X Q, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chemistry, 1995, 53(95): 285-293. ( 0) 0)
|
| [15] |
Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1970, 227(5259): 680-685. DOI:10.1038/227680a0 ( 0) 0)
|
| [16] |
Yen G C, Hsieh P P. Antioxidative activity and scavenging effects on active oxygen of xylose-lysine maillard reaction products. Journal of the Science of Food & Agriculture, 1995, 67(3): 415-420. ( 0) 0)
|
| [17] |
Wang J, Wang Y, Dang X, et al. Housefly larvae hydrolysate: orthogonal optimization of hydrolysis, antioxidant activity, amino acid composition and functional properties. Bmc Research Notes, 2012, 6(1): 1-10. ( 0) 0)
|
| [18] |
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for Superoxide Dismutase. European Journal of Biochemistry, 1974, 47(3): 469-474. DOI:10.1111/ejb.1974.47.issue-3 ( 0) 0)
|
| [19] |
Byun H G, Lee J K, Park H G, et al. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochemistry, 2009, 44(8): 842-846. DOI:10.1016/j.procbio.2009.04.003 ( 0) 0)
|
| [20] |
Zhuang H, Tang N, Dong S T, et al. Optimisation of antioxidant peptide preparation from corn gluten meal. Journal of the Science of Food & Agriculture, 2013, 93(13): 3264-3270. ( 0) 0)
|
| [21] |
Saidi S, Deratani A, Belleville M P, et al. Antioxidant properties of peptide fractions from tuna dark muscle protein by-product hydrolysate produced by membrane fractionation process. Food Research International, 2014, 65: 329-336. DOI:10.1016/j.foodres.2014.09.023 ( 0) 0)
|
| [22] |
Li B, Chen F, Wang X, et al. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chemistry, 2007, 102(4): 1135-1143. DOI:10.1016/j.foodchem.2006.07.002 ( 0) 0)
|
| [23] |
Ren Y, Wu H, Li X, et al. Purification and characterization of high antioxidant peptides from duck egg white protein hydrolysates. Biochemical & Biophysical Research Communications, 2014, 452(4): 888-894. ( 0) 0)
|
| [24] |
Chen N, Yang H, Sun Y, et al. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides, 2012, 38(2): 344-349. DOI:10.1016/j.peptides.2012.09.017 ( 0) 0)
|
| [25] |
Kitts D D, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Current Pharmaceutical Design, 2003, 9(16): 1309-1323. DOI:10.2174/1381612033454883 ( 0) 0)
|
| [26] |
Chen C, Chi Y J, Zhao M Y, et al. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids, 2012, 43(1): 457-466. DOI:10.1007/s00726-011-1102-0 ( 0) 0)
|
| [27] |
Zeng W C, Zhang W H, He Q, et al. Purification and characterization of a novel antioxidant peptide from bovine hair hydrolysates. Process Biochemistry, 2015, 50(6): 948-954. DOI:10.1016/j.procbio.2015.03.007 ( 0) 0)
|
| [28] |
Nimalaratne C, Bandara N, Wu J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chemistry, 2015, 188: 467-472. DOI:10.1016/j.foodchem.2015.05.014 ( 0) 0)
|
| [29] |
Sun Q, Shen H, Luo Y. Antioxidant activity of hydrolysates and peptide fractions derived from porcine hemoglobin. Journal of Food Science & Technology, 2011, 48(1): 53-60. ( 0) 0)
|
| [30] |
Chi C F, Hu F Y, Wang B, et al. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chemistry, 2015, 168: 662-667. DOI:10.1016/j.foodchem.2014.07.117 ( 0) 0)
|
| [31] |
Misbah H, Aziz A A, Aminudin N. Antidiabetic and antioxidant properties of Ficus deltoidea, fruit extracts and fractions. Bmc Complementary & Alternative Medicine, 2013, 13(1): 1-12. ( 0) 0)
|
| [32] |
Je J Y, Qian Z J, Kim S K. Antioxidant peptide isolated from muscle protein of bullfrog, Rana catesbeiana Shaw. Journal of Medicinal Food, 2007, 10(3): 401-407. DOI:10.1089/jmf.2006.169 ( 0) 0)
|
| [33] |
Duan X, Ocen D, Wu F, et al. Purification and characterization of a natural antioxidant peptide from fertilized eggs. Food Research International, 2014, 56(56): 18-24. ( 0) 0)
|
| [34] |
Ranathunga S, Rajapakse N, Kim S K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). European Food Research & Technology, 2006, 222(3): 310-315. ( 0) 0)
|
 2017, Vol. 24
2017, Vol. 24


