2. School of Municipal and Environmental Engineering, Harbin Institute of Technology, Harbin 150090, China
At present, municipal domestic wastewater has become a main constraint on the sustainable development of modern society. Domestic and industrial wastewater is usually disposed of in natural waterways nearby[1]. Untreated wastewater can cause a series of related human diseases and ecosystem destruction. Pollutants in domestic wastewater usually can be categorized into three categories: organics (COD or biochemical oxygen demand), suspended solids (SS), and nutrients (N and P). The biological wastewater treatment method, which is a widely applied technology both at home and abroad, has the advantages of providing a complete treatment, a high rate of organic degradation, no secondary contamination, low energy consumption, convenient operation and so on[2].
COD is a synthetic indicator that represents the degree of organic pollution in water. At present, the potassium dichromate method is generally used to determine the value of COD at home and abroad[3], in addition to other methods, such as spectrophotometry[4] and colorimetry[5]. In this study, we used the fast digestion-spectrophotometric method due to its easy operation, shorter extraction time, and smaller error.
It is an adequate choice for flocculation of suspended solids, somatic cells, and colloidal particles in sewage by microbial flocculants (MBF)[6]. MBF, consists of natural biological macromolecule flocculants, derived from the natural secretion of bacteria during their growth[7]. These metabolic products include protein, cellulose, glycoprotein, polyoses, DNA, and other high-molecular substances[8]. Unlike inorganic/organic composite flocculants, bioflocculants have the advantage of being biocompatible and environmentally friendly[9]. Thus, bioflocculants are regarded as environment-friendly materials[10].
At present, the use of Planococcus sp. has been mainly reported for the production of cold-adapted protease[11-12], detoxification of zearalenone[13], phenol degradation through catechol 2, 3-dioxygenase[14-15], water treatment, etc. Domaradzka et al.[16]treated the naproxen of sewage with Planococcus sp. and found that it showed good treatment effect. Ma et al.[17]obtained a strain named Planococcus sp. from printing and dyeing wastewater. And Ma found that the strain not only had alkali and salt resistance, but possessed also the ability to reduce AQDS and decolorize Orange Ⅰ. Tong et al.[18] found that Planococcus sp. could be used as dominant bacteria to remove COD (removal rate of 64%) in heavy oil wastewater, reaching the national discharge standard. In addition, Ebrahimipour et al.[19] established that the halotolerant Planococcus strain DI could produce extracellular biosurfactants by utilizing crude oil as their carbon source thus being regarded as effective oil-scavengers. So Planococcus strain DI has the ability of degradation. However, there are few reports on the treatment of domestic wastewater with Planococcus representatives.
The scientists and technicians added the dominant strain that might be screened from the nature or may come from recombinant strain into a proper wastewater treatment system, in order to improve the treatment effect of the original system[20]. However, most of the isolated strains had so relatively single function that limited the application range. Thus, the objective of this research was to isolate a bacterium possessing the ability to flocculate and degrade organic material pollutants from the offshore sewage outfall at Weihai International Beach. Moreover, we established a comparatively suitable temperature for the treatment of organic material pollutants in domestic sewage.
2 Materials and Methods 2.1 Sampling and Culture Medium PreparationThe sediment samples were collected from the offshore sewage outfall in Weihai International Beach. The R2A medium[21]consisted of 0.5 g of yeast extract, 0.5 g of peptone, 0.5 g of casein hydrolysate, 0.5 g of glucose, 0.5 g of soluble starch, 0.3 g of sodium pyruvate, 0.3 g of K2HPO4, 0.05 g of MgSO4, 15 g of agar which were dissolved in 1 000 mL aged seawater. The ingredients of the Luria-Bertani media (LB media), utilized herein as a basal medium in 1 000 mL distilled water, were as follows: 10.0 g of beef extract and 10.0 g of peptone. The final pH of the basal medium was adjusted to be within the range 7.4-7.6. The media were autoclaved at 121 ℃ for 20 min.
2.2 Isolation and Preservation of StrainsSediment samples of 50 cm3 were added to 50 mL of sterile sea water, diluted 10-3, 10-4, and 10-5 times according to the decimal dilution method. Coated different dilutions on the plate, and then cultivated at 28 ℃ for four days. Bacteria representatives of predominant morphological types present on the plates were randomly selected and purified on R2A medium. Finally, the isolates were stored at 4 ℃ in a refrigerator.
2.3 Determination of the Flocculation AbilityThe strains isolated from the offshore sewage outfall were screened for the availability of flocculant-producing strains. Kaolin clay was used as the standard substrate for the flocculation activity test in this experiment. Volumes of 1.5 mL of CaCl2 (10%) solution and 10 mL of fermentation broth were added to 1 000 mL of 4 g/L kaolin clay suspension. In the flocculation experiments, a coagulation experimental mixer (ZR4-6) was employed, whose program parameters were adjusted as follows: 400 r/min for 30 s, 150 r/min for 5 min, and 70 r/min for 10 min. After resting for 5 min, the supernatant was collected from the samples. The absorbance value was measured at 550 nm by a spectrophotometer (WFJ2000). The kaolin suspension containing 10% CaCl2 solution without flocculant was used as the control. The flocculation activity (R) is calculated according to the following formula.
| $ R(\% ) = (A - B)/A \times 100\% $ |
where A and B are optical densities of the control and sample respectively, at 550 nm.
2.4 Determination of Optimum Flocculation Condition 2.4.1 Single-factor experimentsThe determination of flocculation conditions adopted a magnetic stirrer, following the same program. 0.3 mL CaCl2 (10%) solution and 2 mL fermentation broth were added to 200 mL of 4 g/L kaolin clay suspension. The optimization of flocculation conditions to improve flocculation effect was carried out single-factor experiments. The effects of flocculation conditions (dosage of the coagulant aids, pH value, dosage of the bioflocculant, concentration of kaolin, and heat stability) on flocculation effect were evaluated by absorbance. The flocculant was used to evaluate the effects of dosage of the coagulant aids (0.5, 1, 1.5, 2.0, 2.5, 3.0, 3.5, and 4 mL), pH value (5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5, and 10.0), dosage of the bioflocculant (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mL), concentration of kaolin on (1, 2, 3, 4, 5, and 6 g/L), and heat stability (20, 40, 60, 80, 100, and 120 ℃) on the flocculation rate.
2.4.2 Distribution of flocculationAfter three days of cultivation, the fermentation liquid was centrifuged and the supernatant was withdrawn for use. The remaining thalli was washed two times with distilled water and then added to the same volume of distilled water to make a bacterial suspension. The flocculation rate of the fermentation broth, supernatant, and bacterial suspension were determined.
2.5 Treatment Efficiency in COD Degradation TestsThe efficiency of different bacteria in COD removal was studied using simulated wastewater samples. Domestic sewage samples(COD concentrations of 400 and 800 mg/L and COD concentrations under high salt with 400 and 800 mg/L) were artificially mixed respectively. A volume of 100 mL artificial sewage was put into a 250 mL conical flask with 6% concentration of the inoculum that was then placed in a rotary shaker at 28 ℃ with a rotating speed of 120 r/min. The test specimen was obtained by centrifuging the fermentation broth with 8 000 r/min for 20 min. After that, the precipitate was removed and clear supernatant extract was analyzed by the fast digestion spectrophotometric method for determination of COD. The COD removal rate (RCOD) was calculated according to the following formula:
| $ {R_{{\rm{COD}}}}(\% ) = (B - A)/B \times 100\% $ |
where A is the final absorbance, and B is the initial absorbance.
2.6 Treatment Efficiency under Suitable Temperature ConditionsThree temperatures were examined to find out which was the most appropriate temperature for COD degradation and flocculation by the halotolerant strain S2A15.Four types of artificial wastewater were configured and the respectively taken 100 mL was added to the 250 mL conical flask. The conical flasks were incubated at different temperatures (4, 20, 28 ℃) in a rotary shaker with a rotating speed of 120 r/min. Similarly, the fermentation broth was centrifuged for COD detection every two days.
2.7 Effect of Different Salinity on Growth of BacteriaTo investigate the growth ability of S2A15 at different salinity, salinity was respectively changed to 0%, 1%, 3%, 6%, 9%, 12%, 15%, and 18% in the LB medium. An amount of 50 mL of LB medium was transferred into a 150 mL conical flask with 6% inoculum concentration. Then, the conical flasks were placed in a rotary shaker at 28 ℃ with a rotating speed of 120 r/min, and the absorbance of fermentation broth was measured by a spectrophotometer (WFJ2000) every 12 h at 600 nm.
2.8 Effect of Different pH Value on Growth of BacteriaTo investigate the growth ability of S2A15 at different pH value, pH value was respectively changed to 4, 5, 6, 7, 8, 9 and 10 in the LB medium. Cultures were placed in a rotary shaker at 28 ℃ with a rotating speed of 120 r/min, and the absorbance of fermentation broth was measured by a spectrophotometer (WFJ2000) after three days at 600 nm.
2.9 Bacterial Strain IdentificationStrain S2A15 was identified by PCR amplification of the 16S rDNA gene using bacterial universal primers 27F and 1522R. The PCR production was sequenced by Sangon Biotech. The sequence was compared with available sequences in the EzBioCloud (http://www.ezbiocloud.net/) and GenBank databases to find model strains with the highest proportion of similarity and completeness. Further, a phylogenetic tree was constructed by the neighbor-joining algorithm using MEGA software (6.0).
3 Results and Discussion 3.1 Colony MorphologyThe strain S2A15 was isolated from sludge at the offshore sewage outfall at Weihai International Beach by enrichment culture and plate-streaking at 28 ℃. The strain was cultured on LB solid media plates, and the colonies (Fig. 1) were observed after 2-3 days. The colonies formed a flat, smooth, moist, and irregular round edge. The colony characteristics of the strain are presented in Table 1.
|
Figure 1 Colony morphology of the strain S2A15 |
| Table 1 Colony characteristics of the strain S2A15 |
3.2 Growth Curve
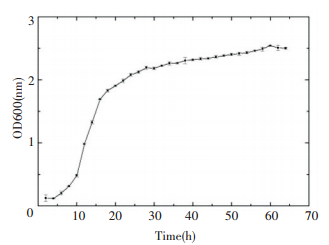
The growth curve that reflects the regularities of growth and reproduction of bacteria is important to the effective use and control of bacteria growth. The growth curve of the strain S2A15 can be divided into four periods:lag phase, logarithmic phase, steady phase, and decline phase. As shown in Fig. 2, the strain S2A15 was still in the lag phase at 0-4 h, in the logarithmic phase at 4-18 h, and in the steady phase in the next 46 h.
|
Figure 2 Growth curve of the strain S2A15 |
The lag phase of this strain was exceedingly short, indicating that S2A15 has the ability to adapt quickly to new environments. The steady phase continued up to 46 h, which is beneficial for the full accumulation of metabolites for strain fermentation. Meanwhile, this culture conditions were suitable for the growth of the strain S2A15.
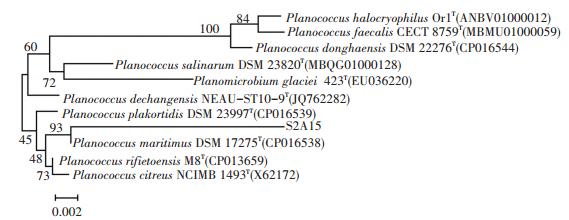
3.3 16S rDNA Gene Sequencing and Similarity AnalysisA strain, which was named as S2A15, that had the ability to combine beneficial traits and potential uses, such as COD degradation, flocculation, and salt and cold tolerance, was isolated from the offshore sewage outfall at Weihai International Beach. A phylogenetic tree was reconstructed based on the 16S rDNA gene sequence of the strain S2A15 and other phylogenetically related strains, which is illustrated in Fig. 3. The sequence similarity of strain S2A15 to the type strain Planococcus maritimus DSM 17275 was 98.8% in the EzBioCloud database (http://www.ezbiocloud.net/). Other related species, such as Planococcus donghaensis, Planococcus halocryophilus, and Planococcus faecalis, exhibited less than 98.6% sequence similarity to strain S2A15. Therefore, strain S2A15 was identified as a Planococcus species based on the similarity of the 16S rDNA gene sequence and its morphology. The nucleotide sequence of 16S rRNA gene was submitted to NCBI GenBank Database under the accession number MG564502.
|
Figure 3 Phylogenetic tree derived from neighbor-joining analysis of partial 16S rDNA sequences |
3.4 Growth under Different NaCl Concentration Conditions
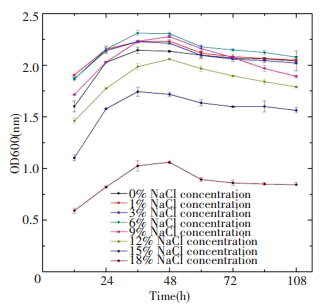
As illustrated in Fig. 4, the strain S2A15 was grown in LB liquid medium with an identical composition, but different concentrations of NaCl were added (0, 1%, 3%, 6%, 9%, 12%, 15%, and 18%). The results indicated that S2A15 was highly tolerant to a salt concentration of up to 15%. Nevertheless, the highest growth rate was established in the medium with 6% NaCl, where the growth was 2.311, higher than that observed in the medium with 15% NaCl. S2A15 successfully survived in the lag phase and entered into the logarithmic phase, indicating that the bacterial enzymatic system adapted effectively to the new environment due to better inoculation amount and activation state of the strain. In addition, since cells of the strain were transferred from a salt-free to salt environment, substances that regulated osmotic pressure, such as amino acids and pyrimidines, were produced in the cells[22].
|
Figure 4 Effect of salinity on the srain S2A15 |
However, the growth of S2A15 was suppressed in the culture medium with 18% NaCl concentration, demonstrating that salt concentrations higher than 15% inhibited the enzymatic activities. Larsen et al.[23] considered that the salt tolerant bacteria that could grow under the conditions of 0-30% NaCl and tolerate up to 18%-20% can be classified as moderately tolerant bacteria. Therefore, the strain S2A15 was identified as a moderately halotolerant microorganism. Halotolerant bacteria can survive well in both high-salt and non-salt environments, as well as maintain their physiological activity, with all these properties depending on their specific cell structure. There are many potential mechanisms to explain salt tolerance. It is possible that Na+ interacts with the cell membrane in a high-salt environment to enhance the mechanical strength of the membranes. It is also probable that S2A15 absorbs K+ into the cell at the same time releases Na+[24].
3.5 Growth under Different pH Value ConditionsThe pH value affected the growth of the strain S2A15, as depicted in Fig. 5. After three days of culture, almost no growth of the strain was observed in the medium with pH 4-5, which had low turbidity. However, when the pH of the medium was elevated to 6, the value of OD600 increased rapidly, which not only showed that S2A15 grew well at that pH value, but also indicated that it was extremely sensitive to acidic conditions. Afterwards, the OD600 increased with the augmentation of pH. At a pH value of 7, the growth of the strain reached its maximum, but at higher pH values, OD600 decreased. Therefore, the strain S2A15 in pH to weak acidic, neutral or alkaline environments, best for growth, too acid environment was not suitable.
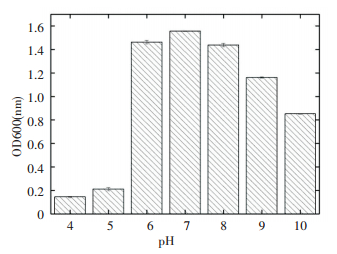
|
Figure 5 Effect of pH on the srain S2A15 |
3.6 Capability of Flocculation
Optimum microbial flocculant doses could be obtained by modificating and regulating the temperature of the culture broth, the dosage of the coagulant aids, pH, etc. The cultural conditions adopted were as follows: 100 mL broth content, pH 7.3-7.4, 1.0% inoculum size, 60 h culture time, 28 ℃ culture temperature, and 120 r/min rotation speed. Under these conditions, the flocculation rate of the strain S2A15 reached 60.19%. The supernatant of this strain showed a flocculation property, indicating that its secretion produced by the S2A15 strain contained effective flocculation components. As can be seen in Fig. 2, according to the growth curve under the condition of 1.0% inoculum size, S2A15 entered the stationary phase from 22 to 60 h. During this stage, the fermentation liquid began to accumulate a large number of useful metabolites, whose quantities reached their maximum. However, after that, the strain began to consume its own metabolites during the decline phase due to the lack of nutrient supply from outside, thus affecting the flocculation rate. The studies of Chen et al.[25], Zhang et al.[26] and Li et al.[27] showed that the late stationary phase was the optimal time for harvesting the product of flocculant-producing bacteria.
3.7 Optimum Flocculation Conditions 3.7.1 Single-factor experimentsOptimization of the flocculation conditions for promotion of the flocculation rate was performed. Using single-factor experiments, the effects of flocculation conditions (dosage of the coagulant aids, pH value, dosage of the bioflocculant, concentration of kaolin, and heat stability) on flocculant production by S2A15 were evaluated. The results presented in Fig. 6 clearly indicate the influence of the dosage of the coagulant aids on the flocculating activity. The increase in the dosage of the coagulant aids during the flocculation process resulted in a continuous rise in the flocculation rate. The highest flocculation rate (65.41%) was reached when added 3 mL the coagulant aids. Within the range of 5-10 of the pH value, the flocculation rate was continuously increased with the elevation of the pH value, but then tended to stabilize at 9 of pH value (Fig. 7). The changes in acidity and alkalinity levels modified the charged state and flocculation ability of the biopolymer and the surface properties of the substances to be flocculated, thus changing the flocculation effect. As can be seen in Fig. 7, through the observation of flocculation process, compared with acidic environment, the microbial flocculant had a faster flocculation speed, bigger flocculation granules and better flocculation effect in the alkaline environment.

|
Figure 6 Effect of dosage of the coagulant aids on flocculating activity |
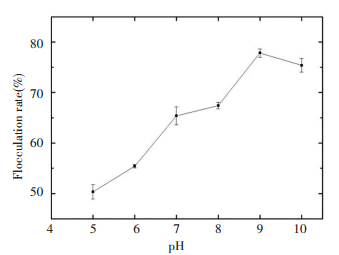
|
Figure 7 Effect of pH on flocculant activity |
As shown in Fig. 8, the highest flocculation rate (77.83%) was reached when added approximately 2 mL of the microbial flocculant, too much or too little of microbial flocculant was found to be ineffective to enhance the flocculation rate. It is noteworthy that there was no flocculation deterioration phenomenon within the experimental settings.

|
Figure 8 Effect of dosage of the flocculant on flocculating activity |
The flocculation rates at different concentrations of kaolin are presented in Fig. 9. With the increase in the concentrations of kaolin during the flocculation process, the flocculation rate was decreased continuously. The flocculation rate was stable within the range 3-5 mg/L of kaolin concentration.
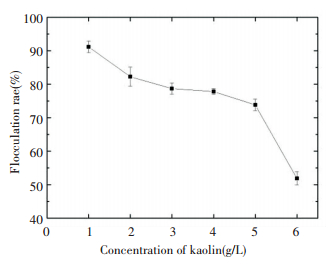
|
Figure 9 Effect of concentration of kaolin on flocculating activity |
Using the above optimal flocculation conditions, the flocculation rate was examined at different temperatures to investigate the thermal stability (Fig. 10). There was only approximately a 5% decrease in the flocculation rate realized by microbial flocculants in the range from 20 ℃ to 120 ℃ after water bath heating for 20 min at different temperatures. Therefore, the flocculants studied herein had good thermal stability. The reason for the decline of the flocculation rate was that some proteins in the microbial flocculants were thermally unstable, which were easily inactivated by high temperature. Therefore, we can speculate that the main component of the microbial flocculant is a saccharide based its thermal stability.
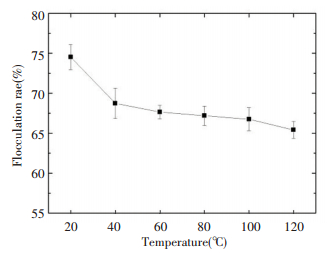
|
Figure 10 Effect of temperature of flocculant on flocculating activity |
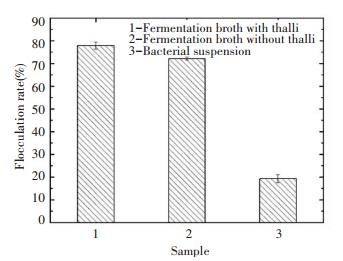
3.7.2 Distribution of flocculation
The flocculation rate of the fermentation broth with thalli was slightly higher than that fermentation broth without thalli, which respectively reached 77.83% and 72.12%. It showed that the main substance of flocculation existed in fermentation broth, which was produced by microbes and secreted to the extracellular. It also indicated that the flocculant also had a small amount to distribute on the surface of thalli. Therefore, it was so convenient in the follow-up experiment or application, because the step of centrifugation could be left out.
3.8 Capability of COD DegradationThe ability of COD degradation at 4, 20, 28 ℃ was investigated. The reason for setting such three temperatures as follows: at 28 ℃ was a universal temperature in the aspect of culturing the bacteria; at 20 ℃, was close to the condition of the sea; at 4 ℃, was set up through research results of other researchers. MO et al.[28] found that Planococcus sp. could live at 4 -37 ℃ belonging to psychrophile bacteria. Liu et al.[29] obtained a Planococcus sp. strain that has ability to treat sewage at low temperatures.
Viewed as a whole, the results indicated that the highest removal ratio of COD was obtained at 28 ℃. COD removal ratio respectively reached 67.28% and 56.30% corresponding to domestic wastewater with 400 and 800 mg/L COD. It may be more suitable for microbial growth and reproduction at 28 ℃, and the activity that bacteria used organic matter were relatively better. All four types of simulated domestic wastewater could be treated by S2A15 at 4 ℃, indicating that the strain could not only survive in a low temperature but also had ability to deal with wastewater to some degree. Consequently, it can be inferred the strain S2A15 is psychrotroph, because its growth and reproduction were not suppressed within the range 0-4 ℃, and the temperature of its maximum growth reached values above 20 ℃.
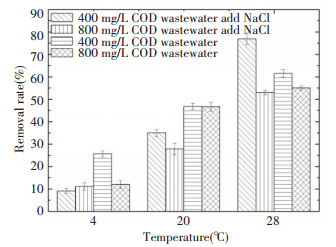
As illustrated in Fig. 11, the removal ratio of domestic wastewater with 400 mg/L COD was almost better than 800 mg/L COD whatever the types of the temperature they were at.
|
Figure 11 Distribution of flocculating activity |
We speculated that the high concentration of COD wastewater could have affected the ability of S2A15 to utilize organic matter. As can be observed in Fig. 12, although the growth of S2A15 in LB medium with 3% NaCl was slightly better than that in the medium without NaCl, the effect seemed to be reverse in the simulated domestic wastewater treatment. At the same temperature, the treatment efficiency of wastewater without salt was higher than wastewater with salt, except for the 400 mg/L COD wastewater with salt at 28 ℃ because the environment, containing a lot of organic matter and salinity, would block metabolic enzyme activities of microorganisms. This resulted in lower organic matter removal rate in the wastewater. This result is similar to the findings obtained by Stewart[30].
|
Figure 12 COD removal rate at different temperatures |
S2A15 was most suitable for treatment of domestic wastewater with 400 mg/L COD without NaCl at 4 ℃, and the removal ratio reached 25.64%. At 20 ℃, the COD removal ratio showed good results for treating 400 and 800 mg/L COD wastewater without NaCl, 46.79% and 46.65%, respectively. At 28 ℃, S2A15 was most suitable for the treatment of domestic wastewater with NaCl and COD of 400 mg/L, where the best removal ratio was achieved (76.9%).
4 ConclusionsIn this study, the strain S2A15 was isolated from the offshore sewage outfall at Weihai International Beach. Based on its phylogenetic characteristics, the isolated strain was identified as a Planococcus species. The strain S2A15 was determined to have the ability to flocculate and degrade organic material pollutants. Meanwhile, it is also salt- and cold-tolerant. This newly identified strain had the ability to reduce 76.9% of COD in domestic wastewater with 400 mg/L COD, with a flocculation ratio reached 60.19%. Moreover, we determined the optimum flocculation conditions using a series of single-factor experiments. The strain could grow in different concentrations of NaCl (from 0 to 18%), whereas the best growth was observed at a salt concentration of 6%. High-salinity wastewater can be directly degraded by halotolerant bacteria, without dilution and pretreatment. Our results show that the strain S2A15 has a good application prospect in domestic water treatment and preserves its beneficial properties for treatments at 4 ℃. Because of these characteristics, it has a wide application value, for example in both salty and cold environments. The treatment ratio of the wastewater may be improved by domestication of the strain or by combinations with other highly efficient strains.
| [1] |
Santhosh P, Revathi D. Studies on laboratory scale sequential batch reactor for treatment of domestic wastewater. Nature Environment & Pollution Technology, 2015, 14(1): 59-64. (  0) 0) |
| [2] |
Gao X, He S, Yang L, et al. Isolation and identification of an efficient psychrotrophic bacterium and its characteristics of organic matter degradation. Acta Scientiae Circumstantiae, 2015, 35(4): 1006-1011. (in Chinese) (  0) 0) |
| [3] |
Yang Qiong, Liu Zhenyao, Yang Jidong. Simultaneous determination of chemical oxygen demand (COD) and biological oxygen demand (BOD5) in wastewater by near-infrared spectrometry. Journal of Water Resource & Protection, 2009, 1(4): 286-289. (in Chinese) (  0) 0) |
| [4] |
Velantika, Purwanto. The textile waste degradation of indigosol blue dye by fention electrical process. Advanced Science Letters, 2017, 23(3): 2239-2242. DOI:10.1166/asl.2017.8714 (  0) 0) |
| [5] |
Yu H B, Wang H, Quan X, et al. Amperometric determination of chemical oxygen demand using boron-doped diamond (BDD) sensor. Electrochemistry Communications, 2007, 9(9): 2280-2285. DOI:10.1016/j.elecom.2007.06.037 (  0) 0) |
| [6] |
Ma Fang, Duan Shuyue, Kong Xiangzhen, et al. Present statue and development trend of studies on microbial flocculants. China Water & Wastewater, 2012, 28(2): 14-17. (in Chinese) (  0) 0) |
| [7] |
Sun J, Zhang X H, Miao X J. Preparation and characteristics of bioflocculants from excess biological sludge. Bioresource Technology, 2012, 126(6): 362-366. (  0) 0) |
| [8] |
Ma F, Wang Q, Meng L, et al. The flocculation efficiency of compound bioflocculant by flocculant producing bacteria. Journal of Harbin Institute of Technology (New Series), 2006, 13(4): 435-438. (  0) 0) |
| [9] |
Guo J Y, Nengzi L C, Zhao J, et al. Enhanced dewatering of sludge with the composite of bioflocculant MBFGA1 and P (AM-DMC) as a conditioner. Applied Microbiology & Biotechnology, 2015, 99(7): 2989-2998. (  0) 0) |
| [10] |
Guo J Y, Chen C. Removal of arsenite by a microbial bioflocculant produced from swine wastewater. Chemosphere, 2017, 8(181): 759-766. (  0) 0) |
| [11] |
Zhang H T, Mu H Y, Mo Q S, et al. Gene cloning, expression and characterization of a novel cold-adapted protease from Planococcus sp. Journal of Molecular Catalysis B-Enzymatic, 2016, 130: 1-8. DOI:10.1016/j.molcatb.2016.04.002 (  0) 0) |
| [12] |
Yadav A N, Sachan S G, Verma P, et al. Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three subglacial lakes of NW India Himalayas. Journal of Basic Microbiology, 2016, 56(3): 294-307. DOI:10.1002/jobm.v56.3 (  0) 0) |
| [13] |
Lu Q J, Bang X C, Chen F. Detoxification of zearalenone by viable and inactivated cells of Planococcus sp. Food Control, 2011, 22(2): 191-195. DOI:10.1016/j.foodcont.2010.06.019 (  0) 0) |
| [14] |
Hupert-Kocurek K, Guzik U, Wojcieszynska D. Characterization of catechol 2, 3-dioxygenase from Planococcus sp strain S5 induced by high phenol concentration. Acta Biochimica Polonica, 2012, 59(3): 345-351. (  0) 0) |
| [15] |
Hupert-Kocurek K, WojcieszyNska D, Guzik U. Activity of a carboxyl-terminal truncated from of catechol 2, 3-dioxygenase from Planococcus sp. S5. The Scientific World Journal, 2014(3): 1-9. (  0) 0) |
| [16] |
Domaradzka D, Guzik U, Hupert-Kocurek K, et al. Cometabolic degradation of naproxen by Planococcus sp. Strain S5. Water Air & Soil Pollution, 2015, 226(9): 297. (  0) 0) |
| [17] |
Ma C, Zhou S G, Lu Q, et al. Decolorization of orange Ⅰ under alkaline and anaerobic conditions by a newly isolated humus-reducing bacterium, Planococcus sp. MCO1. International Biodeterioration & Biodegradation, 2013, 83(9): 17-24. (  0) 0) |
| [18] |
Tong K, Zhang Y H, Liu G H, et al. Treatment of heavy oil wastewater by a conventional activated sludge process coupled with an immobilized biological filter. International Biodeterioration & Biodegradation, 2013, 84(5): 65-71. (  0) 0) |
| [19] |
Ebrahimipour G, Gilavand F, Karkhane M, et al. Bioemulsification activity assessment of an indigenous strain of halotolerant Planococcus and partial characterization of produced biosurfactants. International Journal of Environmental Science & Technology, 2014, 11(5): 1379-1386. (  0) 0) |
| [20] |
Han Liping, Wang Jianlong, Shi Hanchang, et al. Bioaugmentation for removal of recalcitrant organics. Environmental Science, 1999(6): 100-102. (in Chinese) (  0) 0) |
| [21] |
Ma Rui. Isolation and Diversity Analysis of Deep-sea Bacteria In South Atlantic. Harbin: School of Marine Science and Technology, 2013. (in Chinese)
(  0) 0) |
| [22] |
Wohlfarth A, Severin J, Galinski E A. Identification of Nδ-acetylornithine as a noval osmolyte in some Gram-positive halophilic eubacteria. Applied Microbiology & Biotechnology, 1993, 39(4/5): 568-573. (  0) 0) |
| [23] |
Larsen H. Halophilic and halotolerant microorganisms-an overview and historical perspective. Fems Microbiology Letters, 1986, 39: 3-7. DOI:10.1111/fml.1986.39.issue-1-2 (  0) 0) |
| [24] |
Li Jian. Screening, identification and characterization of efficient halo-tolerant bacteria. Northeast Agricultural University, 2013.(in Chinese)
(  0) 0) |
| [25] |
Chen Xiao, Wang Dandan, Li Dongyun, et al. Optimization and its application of cultural conditions for bioflocculant-producing strain. Journal of Nanjing University of Technology (Natural Science Edition), 2009, 31(2): 20-24. (in Chinese) (  0) 0) |
| [26] |
Zhang Xiuzhi, Wang Jing, Zhang Yushan, et al. Isolation and activity exploration of flocculating active marine bacteria HY-20. Ocean Technology, 2010, 29(3): 105-107. (in Chinese) (  0) 0) |
| [27] |
Li Lin, Ma Fang, Zhao Dan, et al. Screening and identification of bioflocculant-producing bacteria and optimization of their culture conditions. China Water & Wastewater, 2015, 31(11): 86-88. (in Chinese) (  0) 0) |
| [28] |
Mo Qingshan, Zhan Huitu, Tian Yao, et al. Screening of cold-adapted producing psychrophiles and their enzymatic properties. Journal of Tianjin University of Science & Technology, 2015, 30(3): 19-23. (in Chinese) (  0) 0) |
| [29] |
Liu Shuangjiang, Liu Zhipei, Jiang Jiatong, et al. Application of planococcus psychrotoleratus in treating sewage at low temperature. CN: 200710065134, 2007.
(  0) 0) |
| [30] |
Stewart M J, Ludwig H F, Ludwig H F, et al. Effects of varying salinity on the extended aeration process. Water Pollution Control Federation, 1962, 34(11): 1161-1177. (  0) 0) |
 2018, Vol. 25
2018, Vol. 25


