2. Key Lab of Non-Ferrous Metal Materials Science and Engineering, Ministry of Education, Central South University, Changsha 410083, China
Zr-Ni based amorphous metal films are widely applied as functional materials, e.g., hydrogen storage materials and sensor materials, due to their unique hydrogen storage properties and better catalytic property[1-2]. However, Zr-Ni alloys alone generally have relatively low glass-forming ability, especially as the Ni concentration increases[3-5], which results in the weakening of corrosion resistance. Therefore, a tertiary element could be added into the Zr-Ni alloys to improve corrosion resistance and glass-forming ability. Ge is such an ideal element for its special metallic and semiconductor properties. The melting point of Ge is lower than that of Zr, Ni and other alloying elements, which can reduce the melting point of the system. For the composition design of amorphous alloy, adding Ge element can not only enlarge the composition range of amorphous alloy, but also effectively improve the corrosion resistance of Zr-Ni alloy[6]. In order to better understand the existing state of the third element Ge in Zr-Ni alloy and the effect of Ge on the phase formation and stability of Zr-Ni alloy, it is necessary to study the phase equilibrium relationship of Zr-Ni-Ge ternary system. Hence, phase equilibria of the Zr-Ni-Ge ternary alloy system are crucial for further understanding the role of Ge element in Zr-Ni based alloys and are of fundamental importance to composition and heat-treatment design.
The research of boundary binary phase relations in the Zr-Ni-Ge ternary system has been perfected, as shown in Fig. 1. In regard to Zr-Ni binary system, in 1961, Kirkpatrick et al.[7] first measured the Zr-Ni binary phase diagram in the whole composition range by means of the optical microscope, X-ray diffraction and optical pyrometer. After that, Nash et al.[8] completely evaluated this system and found 8 stable intermediates: ZrNi5, Zr2Ni7, Zr8Ni21, ZrNi3, ZrNi, Zr7Ni10, Zr9Ni11 and Zr2Ni. There are 11 invariant reactions in the Zr-Ni binary system, including 4 eutectic reactions, 4 eutectoid reactions, 2 peritectic reactions and 1 peritectoid reaction. Recently, Kosorukova et al.[9] re-measured the phase equilibrium relationship of the Zr-Ni binary system, and revised the temperature of each invariant reaction.
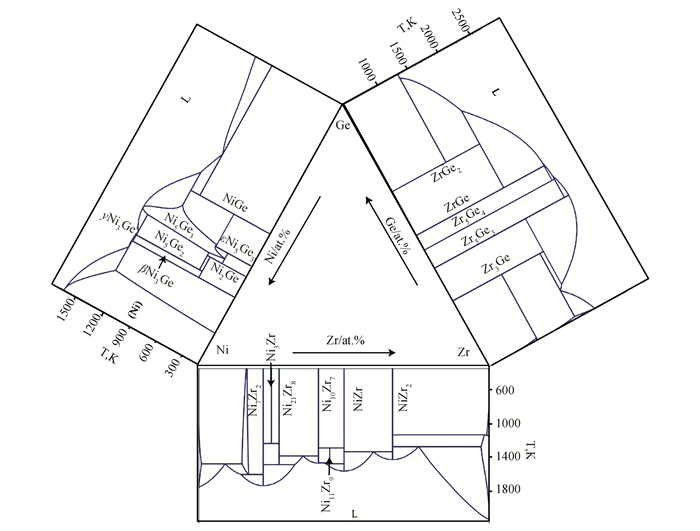
|
Fig.1 Binary phase diagrams the Zr-Ni-Ge ternary system |
As for the Ni-Ge binary system, Ruttewit and Masing [10] evaluated the reported experimental information and constructed the phase diagram. Subsequently, Dayer[11] and Ellner[12] further improved the phase diagram. Based on the previous experiments, Nash[13] carried out thermodynamic optimization of the Ni-Ge system for the first time. Later, Ikeda[14]and Komai[15] found that the solid solubility of βNi3Ge phase reduced with the decrease of temperature. Gel'd[16] and Larsson[17] conducted experimental studies on the dependable evidence of the εNi5Ge3, Ni19Ge12 and Ni3Ge2. It was found that the three independent B8-type intermediate phases were eventually non-stoichiometric Ni5Ge3 solid solution phase. In view of the above problems, Jin et al.[18], based on more recent experimental data, re-optimized the Ni-Ge system, which involved seven intermediate compounds, namely, βNi3Ge, γNi3Ge, δNi5Ge2, Ni2Ge, Ni5Ge3, ε'Ni5Ge3 and NiGe.
The phase equilibrium relationship of the Zr-Ge binary system has been studied by many groups [19-24]. Recently, Sha[24] carried out thermodynamic optimization for the Zr-Ge binary system through First-principle calculation, CALPHAD and experimental methods. As shown in Fig. 1, the Zr-Ge binary system contains five stable intermediate phases Zr3Ge, Zr5Ge3, Zr5Ge4, ZrGe and ZrGe2.
Nevertheless, very little information about phase equilibria and ternary compounds in the Zr-Ni-Ge system was reported besides five ternary intermetallic phases τ1(Zr6Ni16Ge7 [25]), τ2(Zr0.98NiGe2.94 [26]), τ3(Zr3Ni4Ge4 [26]), τ4(ZrNiGe [27]) and τ5(Zr2Ni0.54Ge0.46 [26]) as listed in Table 1, the detailed crystallographic information about τ2(Zr0.98NiGe2.94) is unknown. The present work focuses on measuring the crystallographic data of τ2 and phase equilibrium relationship in the Zr-Ni-Ge system at 973 and 1173 K.
| Table 1 Crystallographic data of solid phases of Zr-Ni-Ge system |
1 Experimental Details
The highly purified zirconium (99.9 wt%), germanium (99.99 wt%) and nickel (99.99 wt%) were used as raw materials. All samples, with a total mass of 6 g, were weighed by using an electronic balance with an accuracy of 0.0001 g.
After being weighed, the raw materials were put into a copper crucible, and then melted in the vacuum non-consumable electric arc furnace. In the melting process, sponge titanium was additionally placed as a getter material. In order to ensure the uniform composition of the alloy, each sample needs to be turned upside and down and melted more than 3 times. After melting, each alloy button was weighed. The mass loss was controlled within 1%.
Each button ingot was wire cut into four parts. After cleaning and removing the oxide layer, each part of the ingot was sealed in a quartz glass tube filled with argon, and then homogenized at 973 K for 100 days and 1173 K for 60 days in a tube furnace, respectively. Then, the tubes containing alloy samples were removed from the furnace and broken immediately so that the alloy was quenched into water at room temperature.
Finally, the constituent phases and their compositions in the annealed samples were investigated by electron probe microanalysis (EPMA) on a JEOL JXA-8800R electron microprobe with an accelerating voltage of 20 kV, a probe current of 1×10-8 A. Constituent phase of the annealed alloy samples were measured by X-ray diffraction (XRD) (Rigaku D-max/2550 X-ray diffractometer, Cu Kα, 40 kV, 250 mA).
Rietveld refinements were carried out with the programs Jade 6[34] and Fullprof-suite[35]. Microstructure characterization using high-resolution transmission electron microscopy (HRTEM) was conducted in a JEM-ARM200 analytic transmission electron microscope working at 200 kV. TEM bright-field (BF) imaging and selected area electron diffraction (SAED) were performed on a JEOL 2010F electron microscope with a field gun at an acceleration voltage of 200 kV.
2 Results and Discussion 2.1 Crystal Structure of Ternary Compound Zr0.98NiGe2.94The phase τ2 was reported firstly by Lysenko in 1978[26], and the composition of τ2 was determined to be Zr11Ni10Ge29. But till now, there has been a lack of detailed crystallographic information on τ2. Alloy S1 with composition of Zr11Ni10Ge29 was fabricated and annealed at 973 K for 100 days. As shown in Fig. 2, Alloy S1 mainly consists of a gray phase. By EPMA measurement, the gray phase contains 19.34 at.% Zr, 21.01 at.% Ni and 59.65 at.% Ge, which should be τ2. In addition, a small amount of white phase is determined to be ZrGe2.

|
Fig.2 BSE (Back-Scattered Electron) image of Alloy S1 |
Then, the S1 sample was ground into powder to further collect its X-ray diffraction pattern and HRTEM data. The experimental parameters of XRD are as follows: the 2 θ range was 5°-120°, each step was 0.02~, and each step was stopped for 2 s. The crystal structure of phase τ2 was indexed with the Jade 6 program[34]. It was found that τ2 has an orthorhombic unit cell and the lattice parameters are as follows: a=11.315(1) Å, b=5.444(9) Å, c=5.355(5) Å. The reflection conditions are as follows:
1) hkl: no conditions;
2) 0kl: l=2n;
3) h0l: h=2n;
4) hk0: no conditions;
5) h00: h=2n;
6) 0k0: no conditions;
7) 00l: l=2n.
The reflection conditions indicate that the possible space group is Pbcm (No.57) or Pca21 (No.29). τ2 is isostructural with LaCrSb3 (Pbcm)[36] by further comparing the powder XRD pattern in the crystallographic characteristics database. So the space group Pbcm (No.57) and crystallographic information of LaCrSb3 were taken as the starting value when the structure refinement of sample S1 was performed by using the Fullprof-Suite program [35]. The formula of τ2 was refined to be Zr0.98NiGe2.94 (19.93 at.% Zr, 20.34 at.% Ni, 59.73 at.% Ge), agreeing well with the measured data, 19.34 at.% Zr, 21.01 at.% Ni and 59.65 at.% Ge. It should be pointed out that, ZrGe2 was input as the second phase during the refinement. The weight percent of the two phases is calculated by quantitative phase analysis in the Rietveld method, which is solved through the Fullprof-Suite program. The powder XRD pattern of the mixture is the weight superposition of the powder XRD patterns of each component phase, and the weight factor of each phase is related to the weight fraction of the phase in the mixture. Therefore, the weight percent of the two phases can be obtained from the relationship between the weight factor and the weight fraction. By this method, the amount of ZrGe2 is calculated to be about 8.8wt% in sample S1.
Fig. 3 shows the calculated and experimental patterns of Alloy S1. The refinement details and crystal data are summarized in Tables 2-4. The final reliability R-factors of Rp=7.46% and Rwp=9.69% indicate highly reliable crystal structure refinement. It can be seen that two disordered sites, Zr 4d and Ge1 4d sites, exist in the lattice and the occupation rate of these two sites are approximately 0.98 and 0.94, respectively. In addition, all bond lengths are reasonable with respect to atomic radii. The crystallographic unit cell of Zr0.98NiGe2.94 is presented in Fig. 4. Neighbors of the Zr and Ni atoms form a cube (CN=11 and 10), and those of the Ge1, Ge2 and Ge3 atoms build heptahedron (CN=6), enneahedron (CN=7) and hendecahedron (CN=8), respectively.
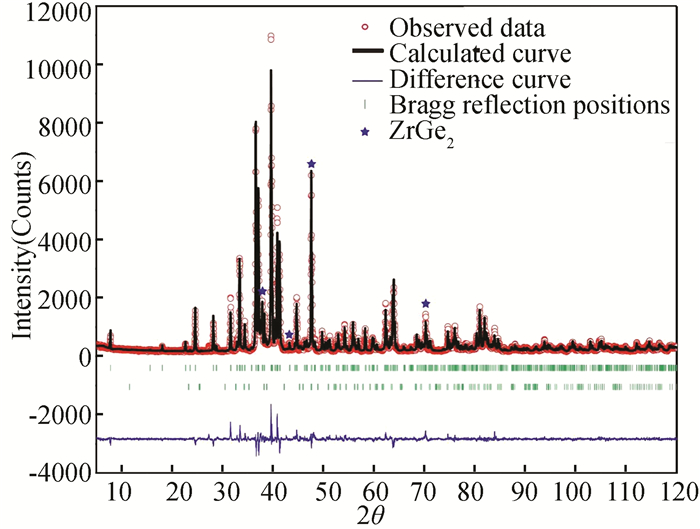
|
Fig.3 Experimental and calculated XRD patterns of Alloy S1 |
| Table 2 Crystal data and structure refinement details for the compounds Zr0.98NiGe2.94 |
| Table 3 Atomic coordinates and temperature factors for Zr0.98NiGe2.94 |
| Table 4 Interatomic distances (δ, Å) and coordination numbers (CN) of the atoms in the crystal structure of Zr0.98NiGe2.94 |
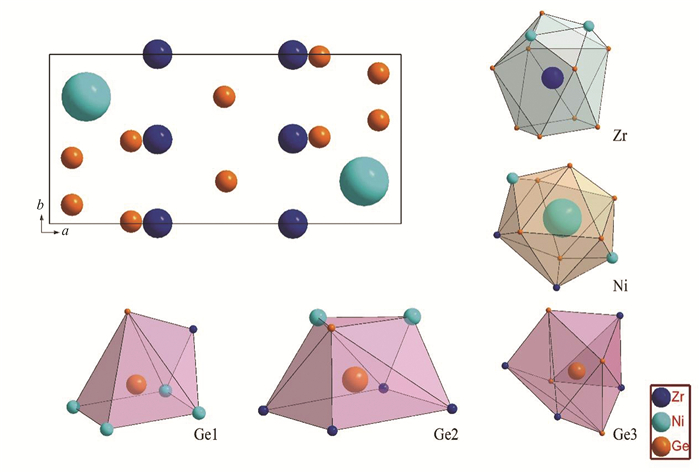
|
Fig.4 Projection of the crystallographic unit cell of Zr0.98NiGe2.94 on ab plane and the coordination polyhedral of the atoms |
TEM was employed to further confirm the crystal structure of the Zr0.98NiGe2.94. The Zr0.98NiGe2.94 powders are smaller than 500 nm, as shown in Fig. 5 (a). The calculated XRD pattern of Zr0.98NiGe2.94 phase based on the crystal structure obtained by refinement is illustrated in Fig. 5 (b). Fig. 5 (c) presents the SAED pattern of a representative powder, the lengths of OA, OB, OC are 3.9525, 5.5096 and 3.23721/nm, respectively, corresponding to the (121) (123) (002) crystal planes of Zr0.98NiGe2.94 phase. This is along [2 1 0] zone axis of the Zr0.98NiGe2.94 phase. Similarly, the SAED pattern in Fig. 5 (d) illustrates the (2 20), (131) and (111) crystal planes along with the [11 2] zone axis of the Zr0.98NiGe2.94 phase. Furthermore, HRTEM image illustrates the interplanar crystal spacing of the (002), (121) and (1 21) crystal planes along [2 10] zone axis is 0.2657, 0.2389 and 0.2358 nm (Fig. 5 (e)). All these results are consistent with the crystal structure of the Zr0.98NiGe2.94 phase. In brief, based on the EPMA and TEM analysis, phase τ2 is refined to be Zr0.98NiGe2.94 with the space group Pbcm (No.57).
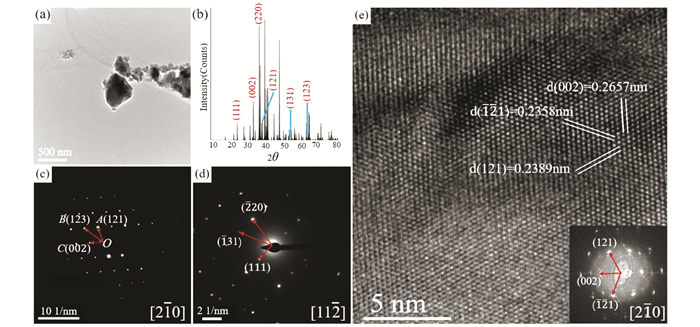
|
Fig.5 (a) Morphology of the Alloy S1 powders; (b) The calculated XRD patterns of Zr0.98NiGe2.94 according to the lattice parameter and interplanar crystal spacing obtained by refinement; (c) SAED pattern of Zr0.98NiGe2.94 along the [2 10] zone axis; (d) SAED pattern of Zr0.98NiGe2.94 along the [11 2] zone axis; (e) HRTEM image of Zr0.98NiGe2.94 along the [2 10] zone axis and its corresponding Fourier transformed pattern |
2.2 Phase Equilibria at 973 K
All alloy samples annealed at 973 K contain two or three phases. According to the phase law, after annealing at 973 K, the samples of Zr-Ni-Ge alloy basically reach or are close to an equilibrium state. The constituent phases and their composition in the annealed alloys are summarized in Table 5.
| Table 5 Constituent phases in annealed Zr-Ni-Ge alloys at 973 K |
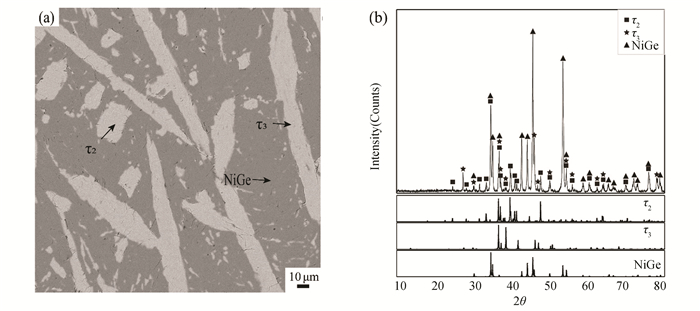
The microstructure of the alloy A4(Zr20Ge50Ni30) annealed at 973 K is illustrated in Fig. 6 (a). It can be seen that alloy A4(Zr20Ge50Ni30) consists of NiGe, τ2 and τ3 with a composition of Zr27.6Ni35.0Ge37.4. Because there are no obvious differences in the color of τ2 and τ3, X-ray diffraction was used to verify the existence of τ2 and τ3, which can be seen in Fig. 6 (b). It means that the alloy A4(Zr20Ge50Ni30) locates in the three-phase region of NiGe+τ2+τ3.
|
Fig.6 (a) BSE image and (b)XRD pattern of Alloy A4 annealed at 973 K for 90 days |
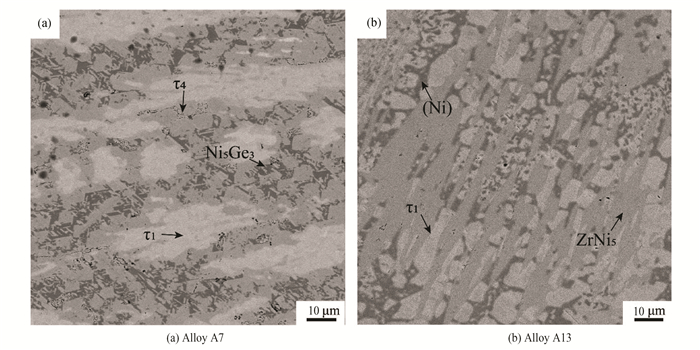
Fig. 7 shows BSE images of alloy A7(Zr20Ge30Ni50) and alloy A13(Zr5Ge10Ni85). The existence of the three-phased equilibria τ1+τ4+Ni5Ge3 and τ1+(Ni)+ ZrNi5 could be identified in alloys A7(Zr20Ge30Ni50) and A13(Zr5Ge10Ni85), respectively.
|
Fig.7 BSE images |
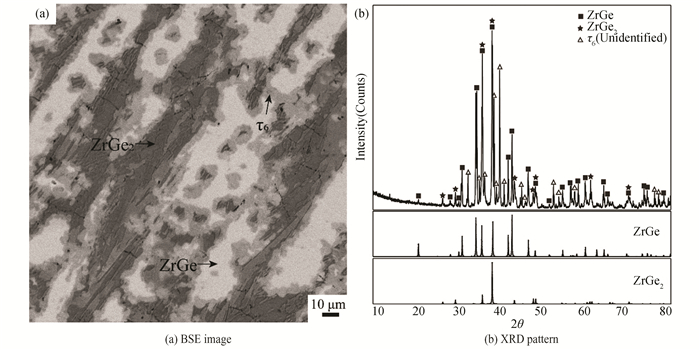
Fig. 8 (a) presents the microstructure of alloy A14(Zr35Ge55Ni10), and it can be seen that alloy A14(Zr35Ge55Ni10) contains ZrGe2, ZrGe, and a new unknown phase with a composition of Zr37.2Ni18.5Ge44.3. After excluding the diffraction peaks of the ZrGe2 and ZrGe phase, some characteristic peaks remained unidentified, as shown in Fig. 8 (b). These peaks must belong to the unknown phase, which is named τ6 hereafter. τ6 was also detected in alloy A15 (Zr40Ge40Ni20). But we failed to prepare a single-phase sample to further collect the X-ray patterns of τ6. The related study of the crystal structure of the phase τ6 will be investigated in future work.
|
Fig.8 Constituent phases in alloy A14 annealed at 973 K for 90 days |
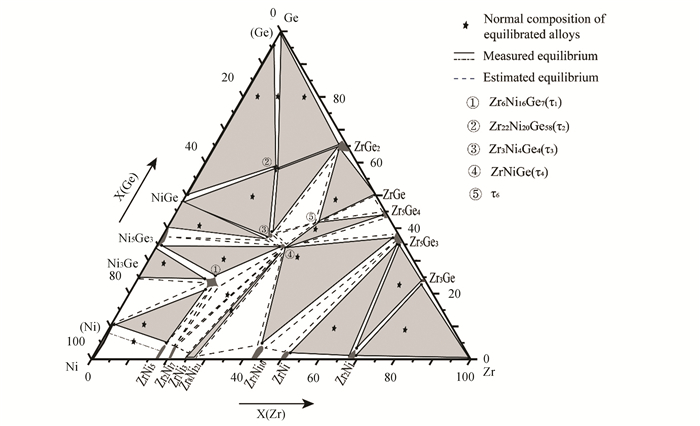
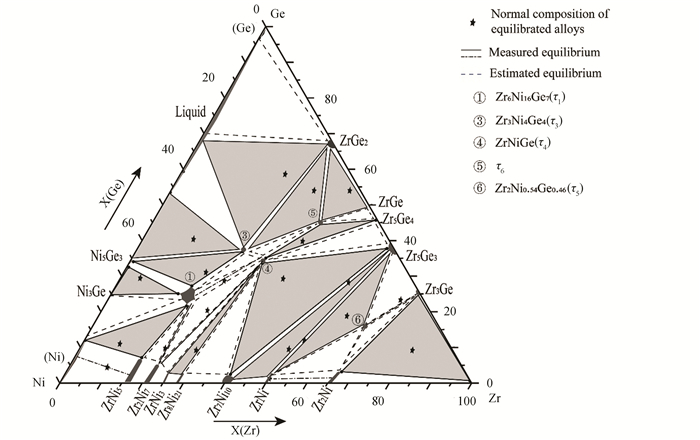
According to the present experimental data, combined with the information of relevant binary systems in the literature, 15 three-phase regions have been measured in the isothermal section of the Zr-Ni-Ge ternary system. In addition, according to the phase law and the measured equilibria phase relations, 11 unmeasured three-phase regions can be extrapolated, as shown in Fig. 9. It should be noted that a new ternary compound τ6 was found.
|
Fig.9 Isothermal section of Zr-Ni-Ge system at 973 K |
2.3 Phase Equilibria at 1173 K
About twenty alloy samples were prepared to determine phase equilibria at 1173 K. The constituent phases and their compositions in the annealed alloys are summarized in Table 6.
| Table 6 Constituent phases in annealed Zr-Ni-Ge alloys at 1173 K |
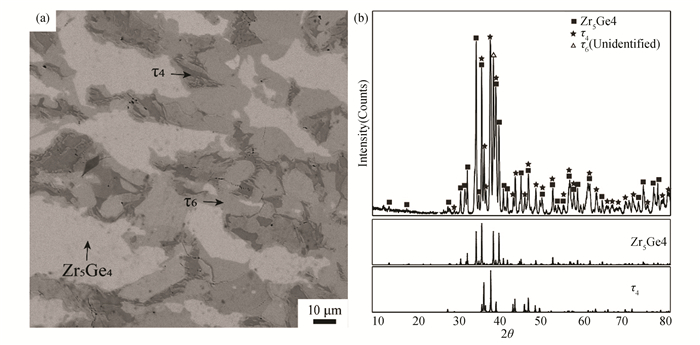
The microstructure of alloy B4(Zr40Ge40Ni20) is presented in Fig. 10 (a). Through EPMA and XRD (Fig. 10 (b)), the white Zr5Ge4 and dark τ4 with a composition of Zr32.5Ni32.4Ge35.1 could be easily observed. Similar to 973 K, an unknown phase with the composition of Zr39.2Ni17.4Ge43.4 similar to that of τ6 was detected. In the XRD pattern of alloy B4(Zr40Ge40Ni20)(Fig. 10 (b)), besides the characteristic peaks of τ4 and Zr5Ge4, there are also some characteristic peaks that cannot be identified with the Jade program. These unknown residual diffraction peaks belong to the new phase τ6.
|
Fig.10 (a) BSE image and (b)XRD pattern of Alloy B4 annealed at 1173 K for 60 days |
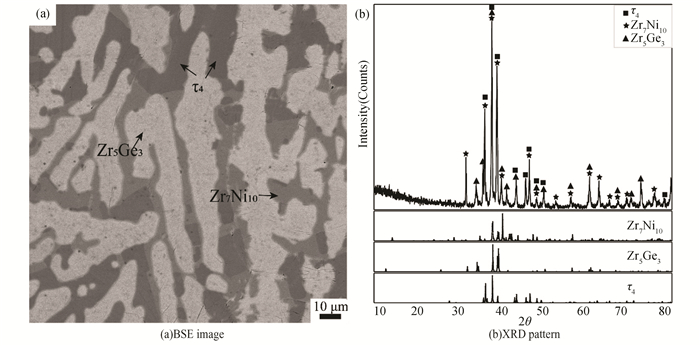
BSE image of alloy B7(Zr40Ge30Ni30) is given in Fig. 11 (a), which consists of dark phase τ4 with a composition of Zr33.2Ni32.8Ge34.0, gray Zr7Ni10 and white Zr3Ge2. This alloy locates in the three-phase region of Zr7Ni10+Zr3Ge2+τ4. The ternary phase τ4 is consistent with that detected by Yarmolyuk [27].
|
Fig.11 Constituent phases in Alloy B7 annealed at 1173 K for 60 days |
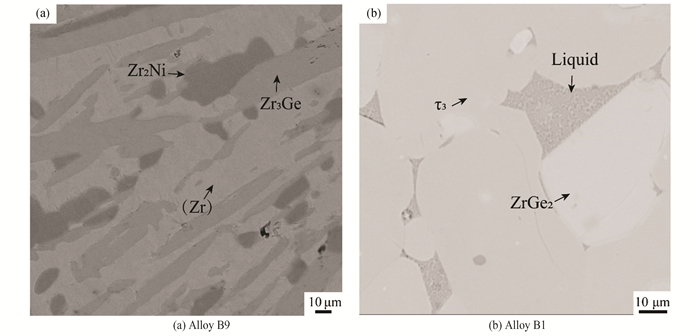
Fig. 12 (a) presents the microstructure of alloy B9(Zr80Ge10Ni10), where phases Zr2Ni, Zr3Ge and (Zr) can be easily identified based on EPMA, which indicated that a three-phase equilibrium Zr2Ni+Zr3Ge+(Zr) exists in the alloy B9(Zr80Ge10Ni10). From Fig. 12 (b), it can be seen that alloy B1(Zr25Ge60Ni15) contains the light gray phase ZrGe2, dark gray phase τ3 and a typical eutectic structure between ZrGe2 and τ3. Because the phases in the eutectic structure are minute, EPMA cannot accurately measure their composition. But referring to the Ni-Ge binary phase diagram and considering the alloy compositions, the alloy B1(Zr25Ge60Ni15) should involve a liquid phase at 1173 K and the liquid phase will solidify into a eutectic structure during water quenching. Therefore, in alloy B1(Zr25Ge60Ni15), the liquid should coexist with ZrGe2 and τ3 at 1173 K. So, the real phase equilibrium might be ZrGe2+τ3+Liquid of alloy B1(Zr25Ge60Ni15) at 1173 K. In this work, the composition of the liquid is estimated by averaging the measured solidified liquid product.
|
Fig.12 BSE images |
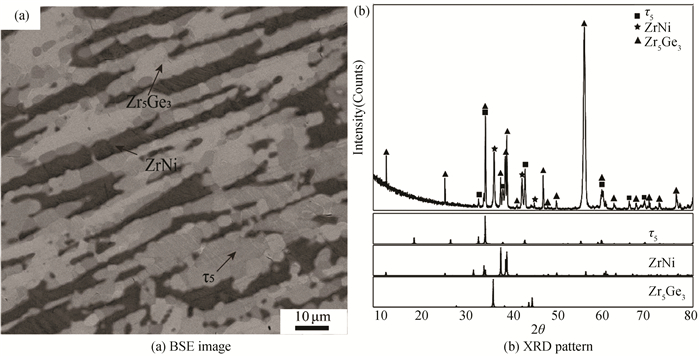
According to the results of EPMA and X-ray diffraction (shown in Fig. 13), alloy B19(Zr60Ge20Ni20) locates in the Zr5Ge3+ZrNi+τ5 three-phase region at 1173 K. Similarly, according to Fig. 14, alloy B22 (Zr30Ge30Ni40) locates in the two-phase region τ1+τ4.
|
Fig.13 Constituent phases in Alloy B19 annealed at 1173 K for 60 days |
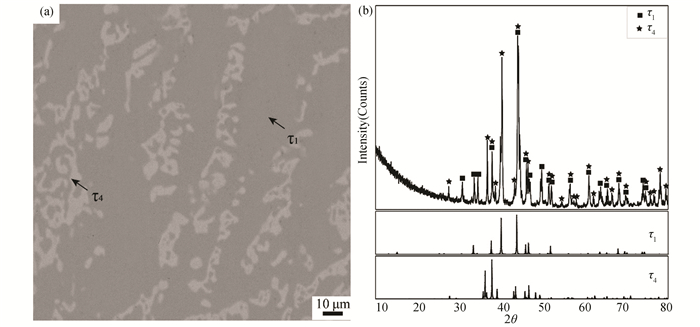
|
Fig.14 (a)BSE image and (b)XRD pattern of Alloy B22 annealed at 1173 K for 60 days |
Based on the experimental results, combined with the phase equilibria at 973 K, the isothermal section of Zr-Ni-Ge system at 1173 K has been determined. Interestingly, τ2 phase disappears at 1173 K. This isothermal section consists of 26 three-phase regions, including 12 measured three-phase regions and 14 estimated three-phase regions, as shown in Fig. 15.
|
Fig.15 Isothermal section of the Zr-Ni-Ge system at 1173 K |
2.4 Comparison of Phase Equilibria at 973 K and 1173 K
Phase equilibria in the Zr-Ni-Ge ternary system at 973 and 1173 K were compared here. Based on Figs. 9 and 15, some distinct differences between these two isothermal sections are presented.
Firstly, when the temperature increases from 973 K to 1173 K, the liquid phase and τ5 appear but τ2 disappears, and the phase relations among (Ge), ZrGe2, τ2, NiGe and τ5 have been changed. It can be seen that phase equilibria (Ge)+τ2, ZrGe2+τ2, (Ge)+ZrGe2+τ2, NiGe+Ni5Ge3+τ3, Zr5Ge3+Zr2Ni+ZrNi and Zr5Ge3+ Zr2Ni+Zr3Ge at 973 K change to (Ge)+ZrGe2, ZrGe2+Liquid, (Ge)+ZrGe2+Liquid, Ni5Ge3+τ3+Liquid, Zr5Ge3+ZrNi+τ5, Zr5Ge3+ Zr3Ge+τ5, Zr2Ni+Zr3Ge+τ5 and Zr2Ni+ZrNi+τ5 at 1173 K.
Secondly, phase relations among Ni5Ge3, τ1, τ3 and τ4 change obviously, i.e., the three-phase equilibria Ni5Ge3+τ3+τ4 and Ni5Ge3+τ1+τ4 at 973 K transform into Ni5Ge3+τ1+τ3 and τ1+τ3+τ4 at 1173 K. In light of phase rule, it is inferred that an invariant reaction Ni5Ge3+τ4↔τ1+τ3 happen at a temperature between 973 and 1173 K.
3 ConclusionsPhase equilibria relation of the Zr-Ni-Ge system at 973 K and 1173 K have been constructed in this work. Crystal structure τ2 was determined, which belongs to space group Pbcm (No.57) with cell parameters a=11.315(1) Å, b=5.444(9) Å and c=5.355(1) Å. Besides the existing five ternary compounds, Zr6Ni16Ge7(τ1), Zr0.98NiGe2.94(τ2), Zr3Ni4Ge4(τ3), ZrNiGe(τ4) and Zr2Ni0.54Ge0.46(τ5), a new ternary phase τ6 with a composition of Zr39Ni18Ge43 was detected. In addition, one four-phase reaction Ni5Ge3+τ4↔τ1+τ3 was deduced, which occurred at 973-1173 K.
| [1] |
Yun D W, Park S B, Park Y. Flexible hydrogen sensor using Ni-Zr alloy thin film. Korean Journal of Materials Research, 2019, 29(5): 297-303. DOI:10.3740/MRSK.2019.29.5.297 (  0) 0) |
| [2] |
Nayebossadri S, Greenwood C J, Speight J D, et al. Thermal and structural stability of Zr-based amorphous thin films for potential application in hydrogen purification. Separation and Purification Technology, 2017, 187: 173-183. DOI:10.1016/j.seppur.2017.06.052 (  0) 0) |
| [3] |
Sahu B P, Dutta A, Mitra R. Influence of composition on nanoindentation response of Ni-Zr alloy thin films. Metallurgical and Materials Transactions A, 2019, 50(12): 5656-5669. DOI:10.1007/s11661-019-05467-8 (  0) 0) |
| [4] |
Wang D P, Wang S L, Wang J Q. Relationship between amorphous structure and corrosion behaviour in a Zr-Ni metallic glass. Corrosion Science, 2012, 59: 88-95. DOI:10.1016/j.corsci.2012.02.017 (  0) 0) |
| [5] |
Sahu B P, Sarangi C K, Mitra R. Effect of Zr content on structure property relations of Ni-Zr alloy thin films with mixed nanocrystalline and amorphous structure. Thin Solid Films, 2018, 660: 31-45. DOI:10.1016/j.tsf.2018.05.050 (  0) 0) |
| [6] |
Zhang J L, Xie X F, Yao M Y, et al. Effect of Ge addition on the corrosion resistance of Z-0.7Sn-0.35Nb-0.3Fe alloy in superheated stem at 400℃. Proceedings of Progress Report on China Nuclear Science & Technology. Beijing: Chinese Neclear Society, 2013.198-205(in Chinese).
(  0) 0) |
| [7] |
Kirkpatrick M E, Bailey D M, Smith J F. The structures of NiZr2, NiZr and their hafnium analogs. Acta Crystallographica, 1962, 15: 252-255. DOI:10.1107/S0365110X62000602 (  0) 0) |
| [8] |
Nash P, Jayanth C S. The Ni-Zr (Nickel-Zirconium) system. Bulletin of Alloy Phase Diagrams, 1984, 5(2): 144-148. DOI:10.1007/BF02868950 (  0) 0) |
| [9] |
Kosorukova T, Ivanchenko V, Firstov G, et al. Experimental reinvestigation of Ni-Zr system. Solid State Phenomena, 2013, 194: 14-20. DOI:10.4028/www.scientific.net/SSP.194.14 (  0) 0) |
| [10] |
Ruttewit K, Masing G. Über die Legierungen des Germaniums mit Wismut, Antimon, Eisen und Nickel. International Journal of Materials Research, 1940, 32(3): 52-61. DOI:10.1515/ijmr-1940-320302(inGerman) (  0) 0) |
| [11] |
Dayer A, Feschotte P. Les systèmes binaires cobaltegermanium et nickele germanium: Étude comparée. Journal of the Less Common Metals, 1980, 72(1): 51-70. DOI:10.1016/0022-5088(80)90252-0(inFrench) (  0) 0) |
| [12] |
Ellner M, Godecke T, Schubert K. Zur struktur der mischung Nickel-Germanium. Journal of the Less Common Metals, 1971, 24(1): 23-40. DOI:10.1016/0022-5088(71)90164-0 (  0) 0) |
| [13] |
Nash A, Nash P. The Ge-Ni (germanium-nickel) system. Bulletin of Alloy Phase Diagrams, 1987, 8(3): 255-264. DOI:10.1007/BF02874917 (  0) 0) |
| [14] |
Ikeda T, Nosé Y, Korata T, et al. The homogeneity ranges of the L12-type intermetallic compounds Ni3Ga and Ni3Ge. Journal of Phase Equilibria, 1999, 20: 626-630. DOI:10.1361/105497199770340617 (  0) 0) |
| [15] |
Komai N, Watanabe M, Horita Z. Interdiffusivity measurements and interface observations using Ni/Ni3Ge diffusion couples. Acta Metallurgica et Materialia, 1995, 43(8): 2967-1974. DOI:10.1016/0956-7151(95)00018-Q (  0) 0) |
| [16] |
Gel'd P V, Levin E S, Zagryazhskii V L. Physicochemical properties and structures of solid and liquid Co5Ge3 and Ni5Ge3. Inorganic Materials and Engineering Transactions, 1979, 15: 14-17. (  0) 0) |
| [17] |
Larsson A K, Withers R. An electron diffraction study of modulated Ni1+xGe B8 type phases. Journal of Alloys and Compounds, 1998, 264: 125-132. DOI:10.1016/S0925-8388(97)00254-5 (  0) 0) |
| [18] |
Jin S, Leinenbach C, Wang J, et al. Thermodynamic study and re-assessment of the Ge-Ni system. CALPHAD, 2012, 38: 23-24. DOI:10.1016/j.calphad.2012.03.003 (  0) 0) |
| [19] |
Rudometkina M V, Seropegin Y D, Shvyryaeva E E. Investigation of the Zr-V-Ge system alloys. Journal of the Less-Common Metals, 1988, 138: 263-269. DOI:10.1016/0022-5088(88)90114-2 (  0) 0) |
| [20] |
Abriata J P, Bolcich J C, Arias D. The GeZr (Germanium-Zirconium) system. Journal of Phase Equilibria, 1986, 7: 43-47. DOI:10.1007/BF02874981 (  0) 0) |
| [21] |
Carlson O N, Armstrong P E, Wihelm H A. Zirconium-Germanium alloy system. 1955. https://www.researchgate.net/publication/255342059_ZIRCONIUM-GERMANIUM_ALLOY_SYSTEM?ev=auth_pub.
(  0) 0) |
| [22] |
Parthé E, Norton J T. Crystal structures of Zr5Ge3, Ta5Ge3 and Cr5Ge3. Acta Crystallographica, 1958, 11: 14-17. DOI:10.1107/S0365110X58000049 (  0) 0) |
| [23] |
Smith J F, Bailey D M. The structures of ZrGe2, HfSi2 and Cr5Ge3. Acta Crystallographic, 1957, 10: 341-342. DOI:10.1107/S0365110X57000985 (  0) 0) |
| [24] |
Sha C S, Zhou L C, Liu S H, et al. Phase equilibria and thermodynamic modeling in the Ge-Zr binary system. Journal of Materials Science, 2011, 46: 1405-1413. DOI:10.1007/s10853-010-4934-1 (  0) 0) |
| [25] |
Spiegel F X, Bardos D I, Beck P A. Ternary G and E silicides and germanides of transition elements. Transactions of the Metallurgical Society of AIME, 1963, 227: 575-579. (  0) 0) |
| [26] |
Lysenko L A, Dzenzelyuk O P. Phase equilibria and crystal structure of compounds in the Zr-Ni-Ge system. Tezisy Dokladov - Vsesoyuznaya Konferentsiya po Kristallokhimii Intermetallicheskikh Soedinenii, 3rd. Summaries of Reports, All-Union Conference on the Crystal Chemistry of Intermetallic Compounds. Lvov, 1978.41-42 (in Russian).
(  0) 0) |
| [27] |
Yarmolyuk Y P, Markiv V Y, Gladyshevskii E I. Compounds with the TiNiSi structure in the systems of two transition metals and either Si or Ge. Visnik L'vivs' kogo (Derzhavnogo) Universitetu, Seriya Khimichna 1969.14-17(in Ukrainian).
(  0) 0) |
| [28] |
Qadri S B, Skelton E F, Webb A W. High pressure studies of Ge using synchrotron radiation. Journal of Applied Physics, 1983, 54: 3609-3611. DOI:10.1063/1.332434 (  0) 0) |
| [29] |
Villars P, Calvert L D. Pearson's Handbook of Crystallographic Data for Intermetallic Phases. Geauga: ASM International, Materials Park, 1991.
(  0) 0) |
| [30] |
Suzuki T, Oya Y, Ochiai S. The mechanical behavior of nonstoichiometric compounds Ni3Si, Ni3Ge, and Fe3Ga. Metallurgical Transactions A, 1984, 15: 173-181. DOI:10.1007/BF02644399 (  0) 0) |
| [31] |
Bhan S, Kudielka H. Ordered bcc-phases at high temperatures in alloys of transition metals and B — Subgroup elements. International Journal of Materials Research, 1978, 69: 333-336. DOI:10.1016/0036-9748(78)90096-0 (  0) 0) |
| [32] |
Castelliz. Kristallstruktur von Mn5Ge3 und einiger ternärer Phasen mit zwei Übergangselementen. Monatshefte für Chemie und verwandte Teile anderer Wissenschaften, 1953, 84: 765-776. DOI:10.1007/BF00902776(inGerman) (  0) 0) |
| [33] |
Sauerschnig P, Grytsiv A, Vrestal J, et al. On the constitution and thermodynamic modelling of the system Zr-Ni-Sn. Journal of Alloys and Compounds, 2018, 742: 1058-1082. DOI:10.1016/j.jallcom.2017.12.012 (  0) 0) |
| [34] |
Zeng W J, Hu K, Liu HS, et al. Crystal structures and elastic properties of Ti(Cu, Pt)2 and Ti(Cu, Pt)3 phases. Transactions of Nonferrous Metals Society of China, 2020, 30: 1839-1848. DOI:10.1016/S1003-6326(20)65343-2 (  0) 0) |
| [35] |
Rodríguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B Condensed Matter, 1993, 192: 55-69. DOI:10.1016/0921-4526(93)90108-I (  0) 0) |
| [36] |
Leonard M L, Dubenko I S, Ali N. Investigation of the ferromagnetism in RCrSb3 (R=La, Ce, Pr, Nd). Journal of Alloys and Compounds, 2000, 303: 265-269. DOI:10.1016/S0925-8388(00)00671-X (  0) 0) |
 2023, Vol. 30
2023, Vol. 30


