2. Science and Technology on Material Performance Evaluating in Space Environment Laboratory, Harbin Institute of Technology, Harbin 150001, China;
3. Radiation and Space Materials Laboratory, Tomsk State University of Control Systems and Radio-Electronics, Tomsk 634050, Russia;
4. Department of Material Science and Technology, Nanjing University of Aeronautics & Astronautics, Nanjing 210016, China
Silica particles exhibit unique optical, electrical, and magnetic properties that can be exploited for various applications. For instance, silica materials can employed in aerospace industry, particularly for the radiation-protective glass covers of solar batteries used in spacecraft. Silica offers excellent resistance to the harsh conditions of space, including intense radiation and extreme temperatures. In addition, silica is also being investigated for use as a pigment or filler in thermal-control coatings for spacecraft[1-4].These pigments or fillers can be nano- or microparticles, or even hollow microspheres[5-6]. Potential applications for hollow silica particles may include the following areas: medicine, fillers in composite materials, adsorbents, catalysts, paints, dye-sensitized solar cells[7-10].
Radiation exposure can induce defects within materials, impacting their structure, electronic properties, and overall performance. Therefore, investigating the mechanisms of defect formation under radiation becomes paramount for the development of radiation-resistant materials. In nanostructured particles, the presence of a large number of grain boundaries and surfaces exacerbates radiation susceptibility. These defects act as atom sites for the formation of radiation-induced defects, compromising the material's stability. Understanding the defect-formation mechanisms in these materials is critical for tailoring their properties and enhancing their radiation tolerance. Radiation-induced defects can manifest in various forms, including vacancies, interstitials, and defect clusters. Each type of defect can affect the material's properties differently, necessitating a thorough understanding of their formation and evolution under radiation exposure. Additionally, the energy and type of radiation (e.g., protons, electrons, or heavy ions) influence the defect formation process. The study of radiation-induced defect formation also holds significant implications for the development of protective coatings for spacecraft. By understanding the defect formation mechanisms, researchers can design coatings that minimize defect formation and enhance radiation stability.
Silicon dioxide can host light absorption centers that arise due to point defects associated with silicon, oxygen, or diverse impurities. The presence of defects adjacent to the silicon-silicon bond[11-12] gives an absorption band at 7.6 eV, unpaired electron on three-coordinate silicon (≡Si·)[13-18] leads to absorption near 6.3 eV (E's1), 6.02 eV (E's2), 4.7 eV (E's3), which has the nature of surface centers. Absorption centers of the anion sublattice can appear in the form of asymmetrically oxygen vacancy with positive charge E'γ[13-18] absorbing quanta of light with energy 5.75-5.85 eV, hole attached by oxygen vacancy leads to the formation of a defect Е'δ[13-20] in this case, the absorption of light will be close 5.6 eV. The band 5.65 eV is determined by silane groups[21].
Hydrogen that is confined by an oxygen vacancy Е'β can give an absorption level with energy of 5.4 eV, the same absorption is typical for ≡Si-O-O·-peroxide radical[13-18, 22]. Wide absorption band B2-5.05-5.16 eV due to decoordinate silica, single or double oxygen vacancy[15, 23-24]. The band near 4.8 eV can be determined one of three types of defects a non-bridging oxygen hole center (≡Si-O·) or a peroxidation radical (≡Si-OO·) or an interstitial ozone O3int[25].
Some authors believe that impurity ions may be responsible for absorption with energies in the range of 3.80-2.75 eV: at high energy, the bands can be determined interstitial chlorine (Cl2int) or peroxide silicon ≡Si-O-O-Si≡ or dicoordinated germanium[25]. In the middle energy range absorption bands may be related to dicoordinated silicon or dioxasilyrane group (≡Si-O)2Si(O2)[25-26]. Absorption near 2.90-2.75 eV may be due to the replacement of silicon cations by aluminum ions[24-26].
The absorption band with 2 eV is determined non-bridging oxygen hole center (≡Si-O·), peroxy radicalgive an absorption band near 1.97 eV[25]. Various molecular vibrations of interstitial oxygen O2int lead to absorption at 1.62 and 0.975 eV[25], for OH-groups at 0.87, 0.64, and 0.55 eV, oxygen molecules О2-, СО and СО2 at 0.76 eV[27].
Hollow silicon dioxide particles can be produced in various ways: synthesis method on emulsion droplets[28-31], layer-by-layer hard-template[32-36], spray method[37-38] as well as thermal plasma sintering[39-40].
The reflective spectra of SiO2 nanosphere, submicrosphere, microsphere, submacrosphere, micro- and nanocrystals powders in the 200-2500 nm range, both before and after protons irradiation are analyzed in this research paper. The impact of size particles on X-ray photoelectron spectra and the deterioration of their optical characteristics under proton radiation were assessed.
1 Experimental 1.1 Sample PreparationThis experiment used analytical-grade, untreatment chemical reagents throughout. Aladdin Chemistry Company provided the 99.8% pure silicon dioxide micro- and nanopowders. Submacropowders were bought from the company Sinosteel Corporation.
Obtaining hollow particles of silicon dioxide can be carried out by deposition of tetraethoxysilane on a solid template, followed by its removal[34-35]. For nanoscale polystyrene beads[36] in 500 mL three-necked flask poured 270 mL deionized water, where 30 mL of styrene and 4.5 g of polyvinylpyrrolidone with a molecular weight of 30000 were added. The solution was mixed at a speed of 300 r/min at a temperature of 80 ℃ for a duration of 30 min. Then, an aqueous solution of ammonium persulfate was added in a ratio of 1.2 g to 72 mL. After that, the mixture was stirred for 9 h at 80 ℃ until a white solution containing polystyrene nanoparticles formed.
To produce polystyrene submicro- and microballs, 300 mL of ethanol was poured into a 500 mL three-necked flask, then, 30mL of styrene and 0.12 g polyvinylpyrrolidone were added to the flask. The solution was mixed at the speed of 300 r/min and heated to 80 ℃ within 30 min. After that, aqueous solution of ammonium persulphate were prepared, one by adding 36 mL of deionized water to 2 g of ammonium persulphate to obtain microbeads, and another using 0.06 g of ammonium persulphate to obtain submicrobeads. Further mixing should be heated no more than 75 ℃ for a duration of 3 h until a white solution was formed. Then the solution was washed 2 times with 500 mL of ethanol, centrifuged at 2000 r/min twice, after each stage, the solution was exposed to ultrasonic treatment. 3 mL γ-aminopropyltriethoxysilane was added to the final solution.
In order to create hollow silicon dioxide particles, the following volumetric ratios of polystyrene beads, ethanol, distilled water, ammonia solution and tetraethoxysilane were added to the solution: 5∶40∶10∶5∶1. Then the resulting solution was mixed for 2 h at a temperature of 50 ℃. After the solation was washed with ethanol and water three times and centrifuged at 3000 r/min after each washing. The final process involved drying the solution and subjecting it to three stages of heat treatment at 200, 300, and 500 ℃. The yield was white and mealy-loose powder.
1.2 CharacterizationThe diffractometer Philips X'Pert PRO MRD (V=40 kV, I=40 mA, CuKα=1.5405) was used for the X-ray diffraction (XRD) analysis. The Helios NanoLab 600i and OXFORD MX2600FE scanning electron microscopes (SEMs) were used to examine the surface morphology of the powders. Using the AutoSorb 6iSA technique, specific surface area of the powder was evaluated by Brunauer-Emmet-Teller (BET) method.
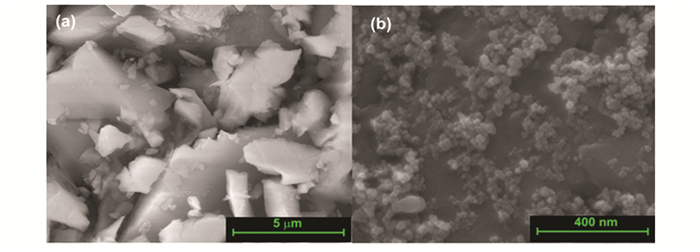
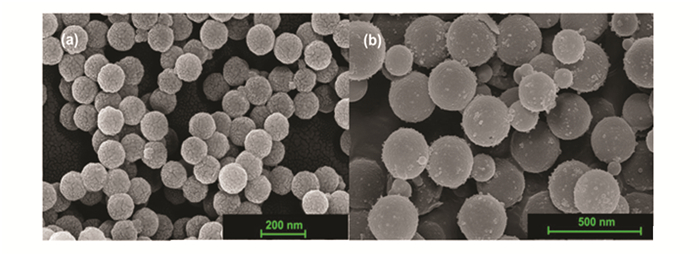
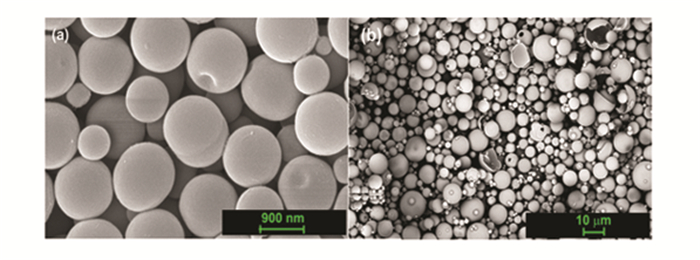
Scanning electron microscopy micrographs (Figs. 1-3) revealed that spherical silica particles, ranging in size from 100 to 10000 nm, are produced throughout the processing. Hollow particles with a size of 100 nm are designated as nanospheres particles, 300-500 nm as submicrosphere particles, 1000-3000 nm as microsphere particles, 7000-13000 as submacrosphere particles. About 90% of the particles are spherical; the remaining particles are either pieces of spheres or are bound together.
|
Fig.1 SEM images of solid (a)micro- and (b)nano-particles of silicon dioxide |
|
Fig.2 SEM images of (a)nano- and (b)submicro-spheres of silicon dioxide |
|
Fig.3 SEM images of (a)micro- and (b)submacro-spheres of silicon dioxide |
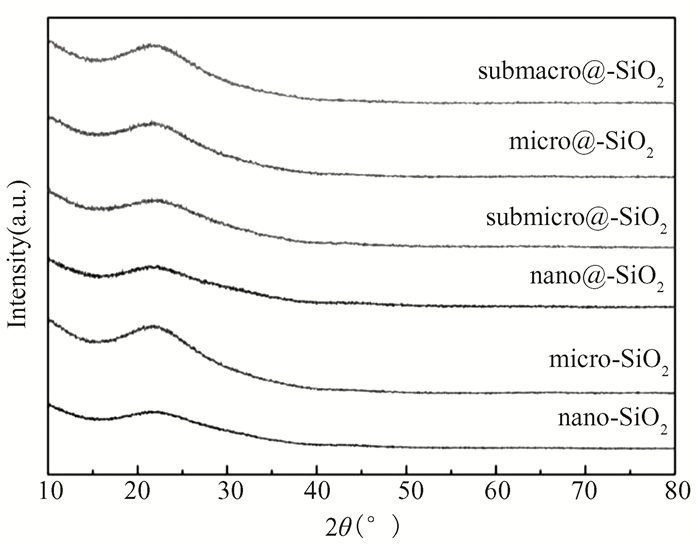
The smallest SSA value (7 m2·g-1) is typical for SiO2 solid microsized particles. SSA of the SiO2 hollow particles has exhibited the values from 25 to 100 m2/g (as shown in Table 1). The obtained XRD spectra (Fig. 4) demonstrate that the amorphous structure of nanoparticles, nanospheres, submicrospheres, microspheres, and submacrospheres is identified by a blurred peak at around 21°.
| Table 1 Characterization of powders by average size distribution and specific surface area |
|
Fig.4 XRD patterns of SiO2 microcrystal, nanocrystal, nanosphere, submicrosphere, microsphere, submacrosphere particles powders |
Samples were created by depositing pastes onto an aluminum disk substrate with a fluted bottom in order to assess the optical characteristics and radiation stability of the powders. The samples' dimensions were as follows: the diameter was 17 mm and height was 2 mm. Deionized water and 50% volume powder were used to make pasta. The substrates were filled with the paste. Following that, the samples were dried at 60 ℃, ensuring their smooth, fracture-free surface remained intact.
The Perkin Elmer Lambda 950 spectrophotometer with a scanning rate of 5 nm/s and a wavelength range of 250 to 2500 nm was used to measure the reflectance spectra of the samples. The X-ray photoelectron spectra (XPS) were measured on ESCALAB 250Xi instrument using an Al-Kα X-ray source at a power of 250 W. The samples were subjected to an initial vacuum of 5×10-5 Pa, followed by an irradiation with protons at an energy of 100 keV fluence 5×1015 cm-2 and a flow rate of 5×1011 cm-2·s-1 in vacuum 2.5×10-4 Pa.
The value of solar absorptance of the samples was calculated in accordance with ASTM (E490 and E903-00a-96) standard. The ρλ is defined as an average of three experimental points.
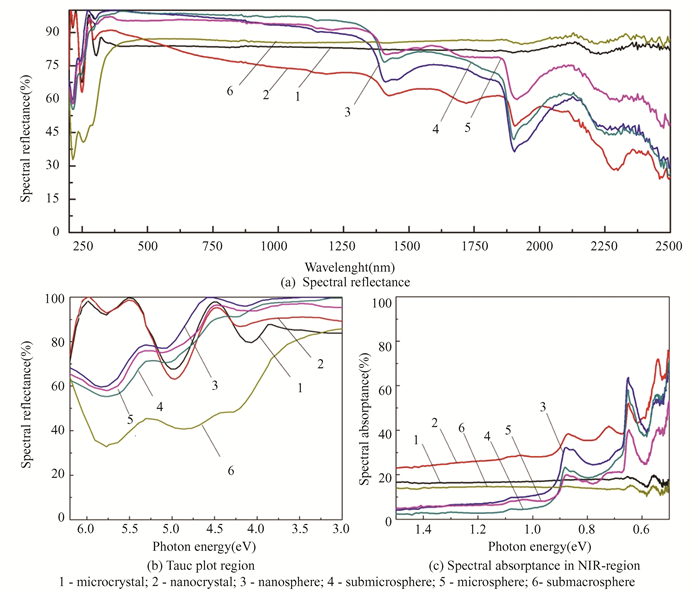
2 Results and Discussion 2.1 Optical Properties of SiO2 Hollow Particles with Different SizeTwo distinct "gaps" are observed in the diffuse reflectance spectra of SiO2 hollow nano-, submicro, micro-, and sub-macroparticles in the region up to 500 nm, as well as for micro- and nanoparticles: a decrease in reflectance to 70%-80% is evident (as shown in Fig. 5), while for submacroparticles, a decrease up to 45% is registered.
|
Fig.5 Optical properties of SiO2 |
Depending on the kind of particle, the reflectance of hollow particles ranges from 30%-45% in the near-IR region and progressively drops in the region above 500 nm. Reflectance for micropowders and submacropowders is 80%-85% at wavelengths higher than 300 nm. Absorption bands corresponding to gases CO, CO2, O2 and OH-groups chemisorbed on powder surfaces are also detected in this spectrum region.
The identical absorption bands at 5.75, 4.9, 4.1-4.2 eV and bands of chemisorbed gases at 0.86, 0.76, 0.64 and 0.55 eV are observed in the absorption spectra of hollow particles shown in Fig. 5(b). The band at 4.9 eV represents defects on non-bridge oxygen atoms (≡Si-O·), while the band at 5.75 eV is the absorption band for silicon dioxide owing to the absorption of surface center (Е'γ), and the band at 4.1 eV is unknown in nature.
The absorption by free electrons is the reason for the absorption spectra (Fig. 5(c)) in the 1.5-0.5 eV region, where the values of the absorption coefficient rise monotonically with increasing wavelength. Since silicon dioxide is a wide-gap dielectric, conductive qualities should not be connected to the existence of free electrons. It might be because of the hollow particles' large specific surface area, which has unsaturated bonds on which different types of defects are localized, and gasses and radicals are adsorbed, resulting in the creation of free charge carriers. There appears to be another absorption band, which could be caused by surface dioxasilyrene groups, based on the absorption band's extended low-energy edge at 3.75-3.8 eV and a noticeable protrusion on it at roughly 2.9-3.0 eV, The photoluminescence results of silica nanoparticles[24-25], which show that the maximum of the emission bands corresponds to 2.41 eV and is connected to dioxasilyrene groups, confirm this.
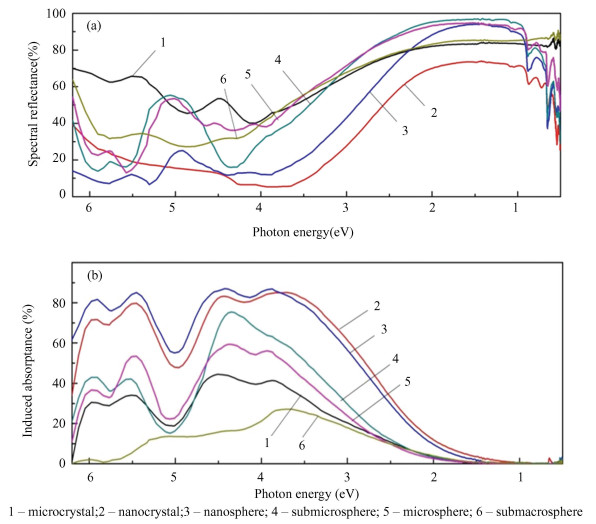
2.2 Effect of Proton Irradiation on Optical Properties of SiO2 Hollow Particles with Different SizeIn the spectral region from 6 to 3 eV, the absorptance of nanostructured particles is significantly higher than the absorptance of micropowders (Fig. 6 (a)). The ratio can be explained by defects on the surface center (E's) that absorb energy. These defects are thought to be three-coordinated silicon atoms with unpaired electrons that are localized at the solid-state boundary. The concentration of these atoms on the formed surface of nanopowders is significantly higher than that of micropowders. In amorphous silicon, a high number of dangling bonds causes a high number of oxygen vacancies to develop, which can then capture thermolized protons and produce E'β -centers. However, in the range from 3 to 1 eV, a high reflectance is observed for hollow particles, but below 1 eV high absorption by hollow particles is again observed.
|
Fig.6 (a) Spectral reflectance and (b)induced absorptance of SiO2 particle after proton irradiation Ep=100 keV, Φp=5×1015 cm-2 |
It can be inferred from the shift in spectral reflectance (Fig. 6(b)) that the diffuse reflectance spectrum of nanostructured materials degrades more during proton irradiation than micropowders do throughout the whole spectral area. However, for hollow particles of submacro sizes, the radiation stability is higher than that of microparticles. The intensity of the induced bands of nanoparticles and nanospheres in the UV region is two times higher than that of microparticles. For submicro- and microspheres, the intensity in the range from 6 to 5 eV is close to microparticles, and from 4.5 to 3.5 eV, their intensity is 20%-30% higher. For submacroparticles, there is a lack of absorption in the high-energy region, in the range from 5 to 3 eV, the intensity is two times less as compared with microparticles, and in the range over 3 eV, they are approximately equal to microparticles. This difference can be caused by different oxygen stoichiometries, which can be analyzed by XPS.
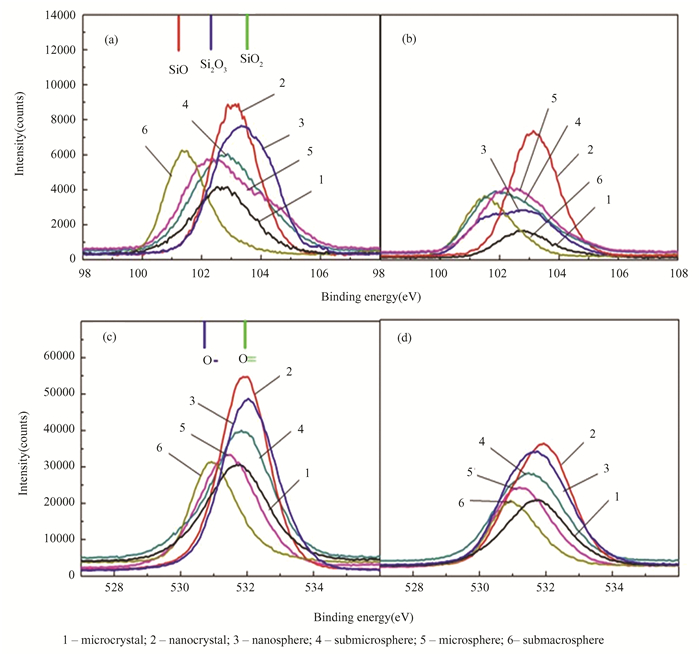
2.3 Effect of Proton Irradiation on XPS Spectra of SiO2 Hollow Particles with Different SizeFor the detection of free oxygen (O2-), non-bridging oxygen (-O-Si), and bridged oxygen-bound defects (Si-O-Si)[41] in silica materials XPS analysis is a suitable technique, because it may reveal both the elements that are present and the other elements to which they are linked. Silicon dioxide and silicon monoxide have fixed energies of 103.3 and 101.9 eV, respectively (as shown in Fig. 7(a)).
|
Fig.7 XPS spectra of SiO2 particle (a, b) spectra of Si2p, (c, d) spectra of O1s after proton irradiation Ep=100 keV, Φp=5×1015cm-2 |
The entire width at half maximum values of 1.5 eV produced the best fit, and this value is comparable to the widths found for silicon dioxide and pure silicon (99.7 eV) [42-43]. In addition to the silicon suboxide SiO, peaks of suboxide Si2O3 are recorded for submacrosphere. Based on the decompositions observed, it was determined that stoichiometry corresponds to SiO2 for particles of a nanoscale range, including nanospheres, while the absence of an oxygen bridge starts for hollow particles. When SiO2 samples are exposed to radiation, the compound's distinctive bands lose intensity due to a high degree of oxygen stoichiometry and shift to a position near 99.7 eV, which represents pure silicon.
This indicates the appearance of defects associated with the formation of non-bridging oxygen. Fig. 7 (c) shows that O1s XPS peaks were fitted to three peaks centered at 530.52, 532.22 and 528.06 eV[44-45]. The shift in the O1s binding energy of nano- and hollow particles compared to microparticles indicates the change in the type of oxygen bonding, which is related to the formation of Si3+. The lattice oxygen in SiO2 and Si2O3 may be responsible for the photoelectron peak observed at around 530.62 and 529.68 eV, respectively. Based on the collected results, the downward departure from oxygen stoichiometry is the reason for the higher radiation stability of hollow particles.
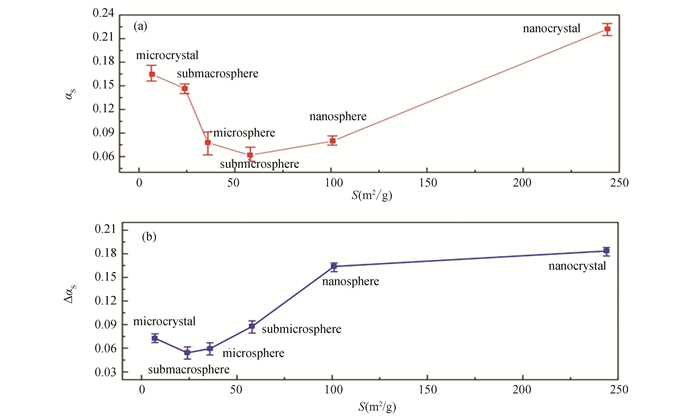
2.4 Solar Absorptance of SiO2 Powders with Different Particle SizesBased on the solar absorptance calculation (as shown in Fig. 8(a)), it was determined that submicrospheres had better initial optical characteristics since their value of αs, which is 0.066, is the lowest among the powders in the selected series. Then, the microspheres and nanospheres, with values of 0.077 and 0.079 are in ascending order of αs. The nanoparticles with αs=0.221 typically exhibit the highest solar absorptance.
|
Fig.8 (a) Solar absorptance of SiO2 powders with different particle sizes and (b)its change in solar absorptance after proton exposure with Ep=100 keV, Φp=5×1015 cm-2 |
Calculation of changes in solar absorptance after proton irradiation (as shown in Fig. 8 (b)) of silicon dioxide samples with different particle sizes showed that the lowest value of Δαs=0.054 corresponds to submacrospheres, followed by microspheres with values of Δαs=0.059, and microparticles with a value of 0.072. The difference in the values of Δαs for microspheres and microparticles, despite the intense absorption peaks in the UV region for hollow particles, is determined by the values of Δρ in the visible region near 480 nm, where the intensity of solar radiation has a maximum, and the values of Δρ for hollow particles were less than for microparticles. For submicrospheres, nanospheres and nanoparticles, the radiation stability is significantly lower.
2.5 Radiation Damage Mechanisms in SiO2 Hollow ParticlesEffects of proton irradiation on silicon dioxide induce a cascade of physical and chemical changes that significantly alter its optical properties. These effects are primarily driven by the high energy of the protons, which interact with the silica atomic structure. Based on standard radiation-induced reactions of amorphous silicon dioxide, processes for the development of defects under the influence of accelerated protons are provided in Ref.[46].
Protons can break down bridge oxygen linkages, resulting in the formation of non-bridging oxygen and non-bridging silicon defects. These defects disrupt the regular Si-O-Si chain and create charge imbalances. The generation of free charge carriers, which might result from the removal of three-coordinate silicon or singly negatively charged oxygen with an increase in charge and hole production as induced reactions. It is feasible for hydrogen to crosslink with silicon or bridging oxygen, producing hydroxyl groups in the process. The material's porous can be increased by hydrogen forming bulk vacancies as a result of local heating. The substance will escape into the vacuum volume if these pores are near the surface. By the same mechanism, a silicon dioxide grain can leave an interstitial. Secondary reactions can be the formation of free charge carriers, which can be formed due to the elimination of three-coordinate silicon or singly negatively charged oxygen with an increase in its charge and the formation of a hole. Crosslinking reactions of hydrogen with bridging oxygen or with silicon and the formation of hydroxyl groups are possible.
While non-bridging oxygen is added to silicon structures, interstitial oxygen can result in the creation of absorption centers based on peroxide radicals. Interstitial neutral oxygen can be added to three-coordinate silicon to generate structures with non-bridging oxygen. When oxygen is knocked out of the connecting oxygen, an oxygen vacancy that can take up a positive charge with protons is created, forming the surface centers E′γ, E′δ, and E′β. There is a chance that an oxygen vacancy will absorb thermolyzed hydrogen, breaking the connection between the silicon atoms and causing three-coordinate silicon to arise.
The disordered structure of glass is caused by the flexible connection≡ Si-O-Si ≡, which is a characteristic of amorphous silica. Such a configuration increases the likelihood of reaction product relaxation and defect recombination on the surfaces of polycrystalline samples. The occurrence of optical absorption centers on the polycrystalline samples' surfaces could be a major factor in how quickly the optical qualities deteriorate when exposed to ionizing radiation.
Compared to microparticles, defects appear more rarely in hollow particles. This is because, during irradiation, the displacement of atoms from charged lattice nodes causes radiation defects to form more extensively in the grain volume of large-grain micropowders. Compared to defects in hollow particles, such defects in the grain volume recover quite slowly. On the other hand, intrinsic defects in the surface and high surface energy could be the main causes of radiation defects in hollow particles (as shown in Fig. 9). Even the smallest impact on the surface causes more structural defects. For zinc oxide and titanium dioxide, an analogous effect of improving the radiation stability of hollow particles relative to solid particles had been investigated[47-48].
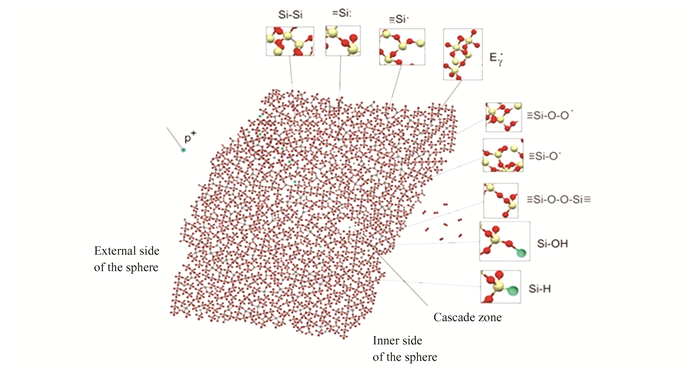
|
Fig.9 Absorption centers formation mechanism in fragment of hollow particle under proton exposure |
Solid microparticles of silicon dioxide with a size of 3000 nm×3000 nm×3000 nm in the shape of a cube, and hollow spheres with a diameter of 3000 nm and a wall thickness of 100 nm, were constructed in the GEANT4 software package (as shown in Fig. 10). Using a dispersed monoenergetic proton beam with a fluence of 5×109 cm-2 and an energy of 100 keV, the beam was angled at a right angle to the surface normal. These forms were put together to make a plate-shaped ensemble. There are 147 particles in all, divided into 7 levels. Each layer is made up of 21 particles, with the center layer being shifted by 1.5 microns in the YZ plane.
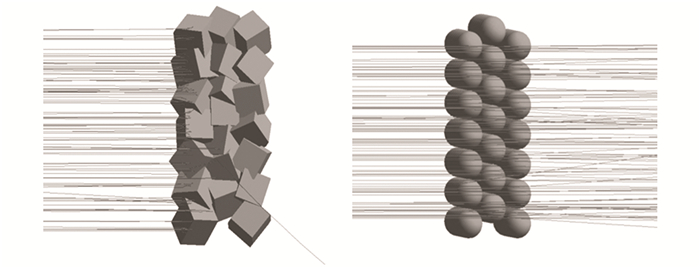
|
Fig.10 Simulation of first 100 protons penetration through SiO2 solid particle with 3000 nm in size and SiO2 sphere with 3000 nm in diameter |
As can be seen from Fig. 7, simulation of the penetration of the first 100 protons with energy of 100 eV through a silicon dioxide microparticle shows that radiation defects are concentrated in the surface layer. Whereas modeling for hollow silicon dioxide particles showed that protons practically do not experience scattering and pass completely through the surfaces. Numerical simulation results showed that the concentration of primary knocked-out atoms for solid particles is 9.274×108 cm-3, whereas for hollow particles, it is 0.0396×108 cm-3. These calculations qualitatively confirm the experimental results.
3 ConclusionsIn conclusion, SiO2 submicrospheres have a higher spectral reflectance than SiO2 micro- and nanoparticles, particularly in the visible range. This implies that hollow submicroparticles will absorb light from the sunlight less efficiently than solid micro- or nanoparticles. It has been established that solid nano- and microparticles are less radiation stable than hollow submicro- and microparticles while exposed to protons. This is because hollow particles have a low concentration of surface defects. The phenomenon could potentially be linked to the relaxation of defects on the vast surface of hollow particles.
| [1] |
Sokolovskiy A, Plis E, Hoffmann R, et al. Study of the optical property degradation of white thermal control coatings under high energy electron irradiation. Surface and Coatings Technology, 2022, 451: 129030-129033. DOI:10.1016/j.surfcoat.2022.129030 (  0) 0) |
| [2] |
Fang M, Lv J. Zn2SiO4 as an ultralow solar absorptive pigment for thermal control coating. Materials Letters, 2019, 255: 126538-126540. DOI:10.1016/j.matlet.2019.126538 (  0) 0) |
| [3] |
Kiomarsipour N, Razavi R S, Ghani K. Improvement of spacecraft white thermal control coatings using the new synthesized Zn-MCM-41 pigment. Dyes and Pigments, 2013, 96(2): 403-406. DOI:10.1016/j.dyepig.2012.08.019 (  0) 0) |
| [4] |
Han Y, Ma W, Xuan Y. Theoretical investigation on degradation behaviors of spectral properties of thermal control coatings induced by charged particles. Applied Surface Science, 2013, 282: 363-369. DOI:10.1016/j.apsusc.2013.05.134 (  0) 0) |
| [5] |
Ye L, Li L, Wang X, et al. Template-free synthesis of uniform hollow silica nanoparticles for controllable antireflection coatings. Ceramics International, 2020, 46(6): 7453-7458. DOI:10.1016/j.ceramint.2019.11.242 (  0) 0) |
| [6] |
Liu C, Feng S, Shi S, et al. Extreme-environment tolerant superhydrophobic montmorillonite/hollow SiO2 composite coating as building radiative cooler for scale energy conservation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 674: 131972-131975. DOI:10.1016/j.colsurfa.2023.131972 (  0) 0) |
| [7] |
Li M, Yuan N, Tang Y, et al. Performance optimization of dye-sensitized solar cells by gradient-ascent architecture of SiO2@Au@TiO2 microspheres embedded with Au nanoparticles. Journal of Materials Science & Technology, 2019, 35(4): 604-609. DOI:10.1016/j.jmst.2018.09.030 (  0) 0) |
| [8] |
Zhao W, Wang W, Meng F, et al. One-pot synthesis of bimetallic Fe/Co incorporated silica hollow spheres with superior peroxidase-like activity. Chinese Chemical Letters, 2023, 34(7): 107858. DOI:10.1016/j.cclet.2022.107858 (  0) 0) |
| [9] |
Jin L, Liu X, Bian C, et al. Fabrication linalool-functionalized hollow mesoporous silica spheres nanoparticles for efficiently enhance bactericidal activity. Chinese Chemical Letters, 2020, 31(8): 2137-2141. DOI:10.1016/j.cclet.2019.12.020 (  0) 0) |
| [10] |
Song L, Zhou N, Gao F, et al. Incorporating organic-modified nano SiO2 for the comprehensive improvement of recycled PET. Chinese Journal of Structural Chemistry, 2023, 42(4): 100042. DOI:10.1016/j.cjsc.2023.100042 (  0) 0) |
| [11] |
Guzzi M, Pio F, Spinolo G, et al. Neutron irradiation effects in quartz: Optical absorption and electron paramagnetic resonance. Journal of Physics: Condensed Matter, 1992, 4(44): 8635-8648. DOI:10.1088/0953-8984/4/44/025 (  0) 0) |
| [12] |
Hosono H, Abe Y, Imagawa H, et al. Experimental evidence for the Si-Si bond model of the 7.6-eV band in SiO2 glass. Physical Review B, 1996, 44: 12043-12045. DOI:10.1103/PhysRevB.44.12043 (  0) 0) |
| [13] |
Zatsepina A F, Kortova V S, YU Biryukova D. Electron-emission activity of defects in surface layers of crystalline and vitreous silica. Radiation Effects and Defects in Solids, 2002, 157: 595-601. DOI:10.1080/10420150215765 (  0) 0) |
| [14] |
Boscaino R, Cannas M, Gelardi F M, et al. ESR and PL centers induced by gamma rays in silica. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 1996, 116(1-4): 373-377. DOI:10.1016/0168-583X(96)00073-0 (  0) 0) |
| [15] |
Skuja L. Optically active oxygen-deficiency-related centers in amorphous silicon dioxide. Journal of Non-Crystalline Solids, 1998, 239(1-3): 16-48. DOI:10.1016/S0022-3093(98)00720-0 (  0) 0) |
| [16] |
Vaccaro L, Morana A, Radzig V, et al. Bright visible luminescence in silica nanoparticles. The Journal of Physical Chemistry C, 2011, 115(40): 19476-19481. DOI:10.1021/jp204350u (  0) 0) |
| [17] |
Nishikawa H, Watanabe E, Ito D, et al. Kinetics of enhanced photogeneration of E' centers in oxygen-deficient silica. Journal of Non-Crystalline Solids, 1994, 179: 179-184. DOI:10.1016/0022-3093(94)90695-5 (  0) 0) |
| [18] |
Pantelides S T, Lu Z Y, Nicklaw C, et al. The E' center and oxygen vacancies in SiO2. Journal of Non-Crystalline Solids, 2008, 354(2-9): 217-223. DOI:10.1016/j.jnoncrysol.2007.08.080 (  0) 0) |
| [19] |
Griscom D L, Friebele E J. Fundamental radiation-induced defect centers in synthetic fused silicas: Atomic chlorine, delocalized centers, and triplet state. Physical Review B: Condensed Matter, 1986, 34: 7524-7533. DOI:10.1103/PhysRevB.34.7524 (  0) 0) |
| [20] |
Chavez J R, Kara S P, Vahneusden K, et al. Microscopic structure of the center in amorphous SiO2: A first principles quantum mechanical investigation. IEEE Transactions on Neclear Science, 1997, 44: 1799-1803. DOI:10.1109/23.658945 (  0) 0) |
| [21] |
Imai H, Arai K, Imagawa H, et al. Two types of oxygen-deficient centers in synthetic silica glass. Physical Review B, 1988, 38(17): 12772-12775. DOI:10.1103/PhysRevB.38.12772 (  0) 0) |
| [22] |
Radtsig V A, Senchenya I N. Hydrogenation of the silanone groups (≡Si-O)2Si=O experimental and quantum-chemical studies. Russian Chemical Bulletin, 1996, 45: 1849-1856. DOI:10.1007/BF01457762 (  0) 0) |
| [23] |
Tsai T E, Friebele E J, Rajaram M, et al. Structural origin of the 5.16 eV optical absorption band in silica and Ge doped silica. Applied Physics Letters, 1994, 64: 1481-1483. DOI:10.1063/1.111891 (  0) 0) |
| [24] |
Griffiths J H E, Owen J, Ward I M. Paramagnetic resonance in neutron-irradiated diamond and smoky quartz. Nature, 1954, 173: 439-440. DOI:10.1038/173439a0 (  0) 0) |
| [25] |
O'Brien M C M. The structure of the colour centres in smoky quartz. Proceedings of the Royal Society A, 1955, 231: 404-414. DOI:10.1098/rspa.1955.0183 (  0) 0) |
| [26] |
Mitchell E W G, Paige E G S. CXI. The optical effects of radiation induced of atomic damage in quartz. The Philosophical Magazine: A Journal of Theoretical Experimental and Applied Physics, 1956, l: 1085-1115. DOI:10.1080/14786435608238193 (  0) 0) |
| [27] |
Burns D A, Ciurczak E W. Handbook of Near-Infrared Analysis. 3rd Edition. Boca Raton: CRC Press, 2001: 814. DOI:10.1201/9781420007374
(  0) 0) |
| [28] |
Park J, Oh C, Shin S, et al. Preparation of hollow silica microspheres in W/O emulsions with polymers. Journal of Colloid and Interface Science, 2003, 266(1): 107-114. DOI:10.1016/s0021-9797(03)00645-3 (  0) 0) |
| [29] |
Jovanovic A V, Underhill R S, Bucholz T L, et al. Oil core and silica shell nanocapsules: Toward controlling the size and the ability to sequester hydrophobic compounds. Chemistry of Materials, 2005, 17(13): 3375-3383. DOI:10.1021/cm0480723 (  0) 0) |
| [30] |
Hah H, Kim J S, Jeon B J, et al. Simple preparation of monodisperse hollow silica particles without using templates. Chemical Communications, 2003, 14: 1712-1713. DOI:10.1039/B301521A (  0) 0) |
| [31] |
Wang Q, Liu Y, Yan H. Mechanism of a self-templating synthesis of monodispersed hollow silica nanospheres with tunable size and shell thickness. Chemical Communications, 2007(23): 2339-2341. DOI:10.1039/B701572K (  0) 0) |
| [32] |
Li W, Sha X, Dong W, et al. Synthesis of stable hollow silica microspheres with mesoporous shell in nonionic W/O emulsion. Chemical Communications, 2002, 38(20): 2434-2435. DOI:10.1039/B206020E (  0) 0) |
| [33] |
Chen M, Wu L, Zhou S, et al. A method for the fabrication of monodisperse hollow silica spheres. Advanced Materials, 2006, 18(6): 801-806. DOI:10.1002/adma.200501528 (  0) 0) |
| [34] |
Xu P, Nan Z, Zhu A, et al. A facile method for preparation of hollow mesoporous silica sphere and its application. Materials Letters, 2017, 205(15): 20-23. DOI:10.1016/j.matlet.2017.06.045 (  0) 0) |
| [35] |
Wang J, Xiao W, Wang J, et al. Hollow mesoporous silica spheres synthesized with cationic and anionic mixed surfactant as templates. Materials Letters, 2015, 142(1): 269-272. DOI:10.1016/j.matlet.2014.11.092 (  0) 0) |
| [36] |
Deng Z, Chen M, Zhou S, et al. A novel method for the fabrication of monodisperse hollow silica spheres. Langmuir, 2006, 22(14): 6403-6407. DOI:10.1021/LA060944N (  0) 0) |
| [37] |
Cheow W S, Li S, Hadinoto K. Spray drying formulation of hollow spherical aggregates of silica nanoparticles by experimental design. Chemical Engineering Research and Design, 2010, 88(5-6): 673-685. DOI:10.1016/j.cherd.2009.11.012 (  0) 0) |
| [38] |
Kim K D, Choi K Y, Yang J W. Formation of spherical hollow silica particles from sodium silicate solution by ultrasonic spray pyrolysis method. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 254: 193-198. (  0) 0) |
| [39] |
Lu C, Fan J, Zhao P, et al. Preparation of hollow silica spheres by DC thermal plasma. Powder Technology, 2014, 266: 210-217. DOI:10.1016/j.powtec.2014.06.045 (  0) 0) |
| [40] |
Fiorilli S, Tallia F, Pontiroli L, et al. Spray-dried mesoporous silica spheres functionalized with carboxylic groups. Materials Letters, 2013, 108: 118-121. DOI:10.1016/j.matlet.2013.06.040 (  0) 0) |
| [41] |
Nesbitt H, Bancroft G, Henderson G, et al. Bridging, non-bridging and free (O2-) oxygen in Na2O-SiO2 glasses: An X-ray photoelectron spectroscopic (XPS) and nuclear magnetic resonance (NMR) study. Journal of Non-Crystalline Solids, 2011, 357(1): 170-180. DOI:10.1016/j.jnoncrysol.2010.09.031 (  0) 0) |
| [42] |
Santucci S, Nardo S, Lozzi L, et al. XPS analysis on SiO2 sol-gel thin films. Journal of Electron Spectroscopy and Related Phenomena, 1995, 76: 623-628. DOI:10.1016/0368-2048(95)02419-0 (  0) 0) |
| [43] |
Hattori T, Nishina T. Studies of SiO2 and Si-SiO2interfaces by XPS. Surface Science, 1979, 86: 555-561. DOI:10.1016/0039-6028(79)90434-5 (  0) 0) |
| [44] |
Zhang R. Structural and optical properties of grey and porous SiO2 nanoparticles. Physica B: Condensed Matter, 2019, 553: 23-25. DOI:10.1016/j.physb.2018.10.027 (  0) 0) |
| [45] |
Khriachtchev L, Nikitin T, Oton C, et al. Optical properties of silicon nanocrystals in silica: Results from spectral filtering effect, M-line technique, and X-Ray photoelectron spectroscopy. Applied Physics, 2008, 104(10): 104316. DOI:10.1063/1.3010304 (  0) 0) |
| [46] |
Li Chundong, Mikhailov M M, Neshchimenko V V. Radiation stability of SiO2 micro-and nanopowders under electron and proton exposure. Nuclear Instruments and Methods in Physics Research B, 2014, 319: 123-127. DOI:10.1016/j.nimb.2013.11.007 (  0) 0) |
| [47] |
Neshchimenko V V, Li Chundong, Mikhailov M M. Radiation stability of TiO2 hollow particles pigments and coatings synthesis by hydrothermal methods from TTIP. Dyes and Pigments, 2017, 145: 354-358. DOI:10.1016/j.dyepig.2017.03.058 (  0) 0) |
| [48] |
Neshchimenko V, Li C, Mikhailov M, et al. Optical radiation stability of ZnO hollow particles. Nanoscale, 2018, 10: 22335-22347. DOI:10.1039/c8nr04455d (  0) 0) |
 2024, Vol. 31
2024, Vol. 31


