2. Medical Device Technology Engineering, AL-Qalam University College, Kirkuk 36001, Iraq;
3. Department of Petroleum Technology, Koya Technical Institute, Erbil Polytechnic University, Erbil 44001, Iraq
This study focused on improving the performance of a flat solar collector by using nanofluid. In recent literature, the researchers used a MWCNT-H2O nanofluid with a diameter of 10-30 nm and a weight concentration of 0.2%-0.4% to increase the collector's efficiency by 83%. In this study, the thermal efficiency of the collector is linearly related to nanoparticle mass flow rate and volumetric concentration. By using a nano-diamond fluid with a concentration of 1% and a efficiency of 69.8% compared to water, the flat solar collector's efficiency was maximized. The study also measured the viscosity of the nanofluid and water using the Brookfield Digital Viscometer DV-Ⅱ+ Pro. The experiments were conducted for two months in Kirkuk's climate, and the margin of error was found. Overall, the results suggest that using nanofluid can significantly improve the efficiency of flat solar collectors.
To improve the performance of a 2 m2 flat solar collector, Yousefi and Veisy[1] used a MWCNT nanofluid with a diameter of 10-30 nm, a weight concentration of 0.2%-0.4%, and a mass flow rate of 0.0167-0.05 kg/s. The flat solar collector's efficiency rose 83% by increasing mass flow rate and nanofluid concentration. An experimental research was carried out on a 0.34 m2 flat solar collector using CNT nanofluid with a diameter of 1 nm and weight concentrations of 0.6%, 0.5%, and 0.4%[2]. At 0.5%, efficiency increased by 39%. In an experimental evaluation of thermal performance in a flat solar collector with an area of 1 m2, Zamzamian et al.[3] employed Cu nanofluids combined with ethanol alcohol with a diameter of 10 nm, volumetric concentrations of 0.2%-0.3%, and mass flow rates of 0.016-0.050 kg/s on a flat solar collector with a 0.67 m2 surface, and a concentration of 0.3% was discovered. Nasrin and Alim[4] evaluated the thermal performance of a 1.51 m2 solar collector. In distilled water, 50 nm Al2O3, 50 nm CuO, and 25 nm TiO2 nanoparticles were used. Laminar flow was created using three nanoparticle concentrations (0.2%, 0.4%, and 0.8%) with a mass flow rate of 4.0 kg/min. The augmentation of surface heat transfer rate was observed with elevated nanoparticle concentration within the base liquid, depending on the type of particles used. Notably, the presence of copper particles resulted in a significantly higher heat transfer rate compared to systems utilizing aluminum oxide and titanium oxide particles. The most notable enhancement, reaching 87.8%, was attained with copper oxide when employed alongside pure water, surpassing the improvement achieved using distilled water, which measured 52.5%.
Moghadam et al.[5] performed an experimental investigation of the thermal performance of a 1.88 m2 solar collector. At a mass flow rates of 1, 2, and 3 kg/min, and a diameter of 40 nm, CuO nanofluid with a mass concentration of 0.4% flows laminarly. The efficiency percentage was found to be 21.8% higher than when using water as the base fluid. Michael and Iniyan[6] observed that a CuO nanofluid with diameters of 0.3 and 0.4 nm, mixed with water as the base fluid and at a volumetric concentration of 0.05%, enhanced the thermal efficiency of a 2.184 m2 solar collector. At 0.1 kg/min, the solar collector's efficiency is 57.98% compared to a forced load. Al2O3 nanofluid was mixed with distilled water with a diameter of 15 nm and volumetric concentrations varying from 0.090696% to 0.1423% and evaluated on a 1.51 m2 flat solar collector. Adjusting parameters and lowering energy generally improves nanofluid efficiency when paired with water. Said et al.[7] evaluated a flat solar collector with an area of 1.84 m2 using a 21 nm TiO2 nanofluid at volumetric concentrations of 0.1% and 0.3%. A laminar flow rate of 0.5 kg/min was achieved. The results showed a 76.6% efficiency improvement compared to water. Polyethylene clay cool PEG stabilized liquid. In an experimental study, a flat solar collector with an area of 1.59 m2 was used, and nanofluid TiO2/EG-water with a diameter of 10 nm and volumetric concentrations were employed to improve solar collector performance (0.5, 0.75, and 1). At a turbulent flow rate of 2.7 kg/min, the efficiency increased 4%-8% compared to water.Salavati Meibodi et al.[8] studied a flat solar collector with an area of 1.59 m2 using nanofluid TiO2 with a diameter of 10 nm and volumetric concentrations of nanofluid(0.5, 0.75, 1) to improve solar collector efficiency (0.2, 0.4 and 0.6). At a turbulent flow rate of 3 kg/min, efficiency rose 23.5% compared to water. Vakili et al.[9] demonstrated that increasing the weight fraction of the nanofluid enhanced collector efficiency, with the highest efficiency observed at a flow rate of 0.015 kg/s for both the base fluid and nanofluids, achieving zero-loss efficiencies of 83.5%, 89.7%, and 93.2% for weight fractions of 0.0005, 0.001, and 0.005, respectively, compared to 70% for the base fluid. Verma et al.[10] studied a flat solar collector with an area of 0.375 m2 to find how MgO nanofluid with a diameter of 40 nm and varying volume concentrations 0.25%, 0.5%, 0.75%, 1.0%, 1.25% and 1.5% at 0.5, 1.0, 1.5, 2.0, 2.5 L/min respectively. At a concentration of 0.75% and a volumetric flow of 1.5 L/min, the collector's heat efficiency rose by 9.34% and its exergy efficiency increased by 32.2% compared to water. At volumetric concentration of 0.75%, the entropy generated is 0.0611 W/K; At volumetric concentration of 1.5%, the entropy generated is 0.1394 W/K and 0.071 W/K, respectively. Jouybari et al.[11] experimentally studied the thermal performance of nanofluid flow through a flat plate solar collector with a metal porous foam-filled channel. SiO2/deionized water nanofluids with volume fractions of 0.2%, 0.4%, and 0.6% were tested. The results showed an improvement of up to 8.1% in thermal efficiency but also an undesirable increase in pressure drop. The Performance Evaluation Criterion (PEC) indicated that as the nanoparticle volume fraction increased from 0.2% to 0.6%, the PEC improved from 1.07 to 1.34 at a flow rate of 0.5 L/min.Researchers found that mixes of (SiO2+H2O), (TiO2+H2O), (Al2O3+H2O), (CuO+H2O), (Graphene+H2O), and (MWCNTS+H2O) had better ratios (5.74%, 6.97%, 10.86%, 16.67%, 21.46%, 29.32%) than water under liquid circumstances. Compared with water as the base fluid and the solar collector's best energy efficiency, SiO2+H2O, TiO2+H2O, Al2O3+H2O, CuO+H2O, Graphene+H2O, and MWCNTS+H2O lowered entropy by 10.04%, 24.49%, 36.84%, 48.32%, 57.89%, and 65.55%, respectively. A 0.8 m2 solar collector was examined for thermal efficiency. Increasing solar collector efficiency can be achieved by mixing water with a TiO2 nanofluid with a diameter of 7 nm. Laminar flow was created using three nanoparticle concentrations (0.2%, 0.4%, and 0.8%) with a flow velocity of 1.5 kg/min. As proven experimentally, increasing the nanoparticle concentration in the base liquid promotes heat transmission, and the type of particle affects the efficiency of the flat surface collector. Efficiency rises by 8% compared to water.Syam Sundar et al.[12] and Zarda et al.[13] tested Al2O3 nanofluids with diameters of 20 nm, at concentrations of 0.1% and 0.3% by volume, and with a flow rate of 5 kg/s on a 2 m2 flat solar collector to increase efficiency. Compared to water, the efficiency was 18% greater. Kiliç et al.[14] investigated a TiO2 Triton X-100 nanofluid, characterized by a particle diameter of 44 nm and a volumetric concentration of 0.2%. This investigation employed a flat solar collector with a surface area of 1.82 m2. The measurements, which pertained to mass-related quantities, were taken in seconds.
Sharafeldin and Gróf[15] conducted a study investigating the impact of varying concentrations of CeO2 nanofluid within water (used as the base fluid). The study focused on a flat solar collector with a surface area of 2.03 m2, and the experiments were carried out at mass flow rates of 0.00167%, 0.0333%, and 0.0666%, respectively. The working fluid mass flux rates were measured at 0.019, 0.018, and 0.015 kg/(s·m2), the results indicated that the utilization of a nanofluid instead of regular water as the base fluid led to a notable enhancement in collector efficiency. Specifically, the researchers observed a linear correlation between the collector's thermal efficiency and both the nanoparticle mass flow rate and the volumetric concentration of the nanofluid. Tong et al.[16] evaluated different concentrations of nanofluids (CuO, Al2O3) in water as the base fluid, with a diameter of 20-40 nm, on a flat solar collector with an area of 2 m2, and using Al2O3 nanofluids of 1.5 vol% instead of water.The results showed that the flat solar collector efficiency were rasied to 21.9%. Water generated the most entropy, followed by 0.1% Al2O3 nanofluid. An Al2O3 nanofluid with a volumetric content of 0.1% and a CuO nanofluid with 0.5% boosted energy by 56% and 49.6%, respectively, compared to water. The flat solar collector's greatest thermal efficiency was achieved using 0.1% Al2O3 nanofluid in water. Nirmala[17] tested ways to improve flat solar collectors. In the sunlight, the distance between the glass and the absorbing plate was 2 cm.At the same time, with the same size as the flat solar collector, a double glass plate and a single glass plate were placed at the same distance from the absorption plate. The study found that the double glass plate doubles the efficiency of the flat solar collector compared to the single glass plate. Choudhary et al.[18] tested a 2.1 m2 flat solar collector, utilizing nanofluid (ZnO) with a diameter50 nm added to a mixture of ethylene clay powder and distilled water. At the flow rate of 150 L/h, the collector's exit temperature is 318.62 K, and the flat solar collector's thermal efficiency increases from 30 to 150 L/h. At the flow rate of 60 L/h and the nanofluid concentration of 1%, the thermal efficiency is 69.24%, which is 19.24% higher than that achieved with distilled water. Okonkwo et al.[19] examined single(Al2O3) and hybrid nanofluids (Al2O3-Fe) using water as the base fluid, in a flat solar collector with an area of 1.51 m2. The hybrid nanofluid lowered flat solar collector thermal efficiency by 1.79% compared to water, while nanofluid (Al2O3) at 0.1% enhanced thermal efficiency by 2.16%. Hybrid nanofluids boost exergy efficiency by 6.9% over solo nanofluids. Alklaibi et al.[20] experimentally studied a flat plate solar collector using nanodiamond (ND) nanofluid under thermosyphon conditions. ND nanoparticles were added to distilled water at concentrations of 0.2% to 1.0%. The study focused on thermal efficiency, exergy efficiency, entropy generation, and heat transfer. The highest thermal efficiency, 69.85%, was achieved with 1.0 vol% ND/water nanofluid, a 12.7% increase over water. Entropy generation dropped from 5.725 W/K with water to 5.541 W/K with 1.0 vol% ND/water nanofluid.
1 Methodology 1.1 Experimental ConfigurationAfter the planar solar collector's design, identification, and planning stages, each component's production techniques were developed, as shown in Fig. 1.
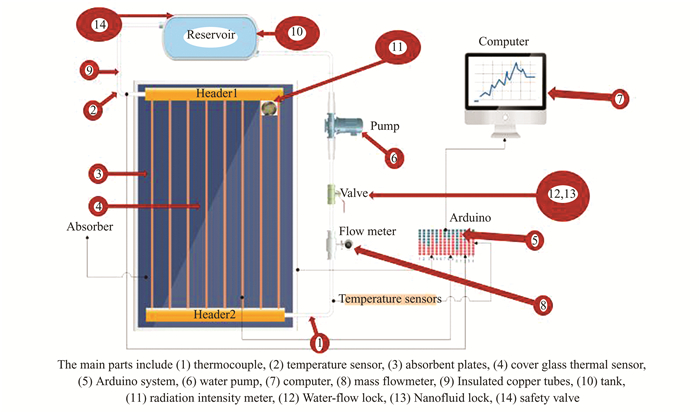
|
Fig.1 Scheme of planar solar collector |
The system's core is a 1 m2 metal box. The shell has three plastic panels, an aluminum skin, and 5 cm of glass fiber between them. Clean glass covers it. The device is fixed and immovable with a 4 mm thickness to suit its large cross-sectional area, and protect it from breakage and weather, while maximizing energy absorbed from the top by allowing solar radiation to penetrate. It reduces thermal losses from the front side of the complex, as well as from the bottom and sides. And it is insulated from heat. Electrically mixing TiO2 and water formed a nanofluid for the second part. The nanofluid ensures water nanoparticle dispersion. The solar collector consistes of copper tubes, each measuring 0.85 m in length.
The header tubes' inner diameter is 22.5 mm, and their thickness is 1 mm. The riser tube's inner diameter is 9.5 mm, and its thickness is 1.5 mm. Pipes linking system components were insulated with two layers of Armaflex insulation to prevent heat loss through the tank walls. German-made 21-inch plastic tubes connected the solar collectors and tanks, with plastic tubes couplings and valves controlling the flow of water and nanofluid. Two quarter-horsepower vertical pumps, each at least 2.5 m tall, moved water and nanofluid through the system, as shown in Fig. 2.
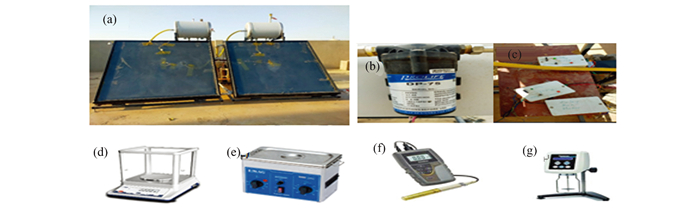
|
Fig.2 The experiment apparatus includes (a) solar collectors, (b) fluid pump, (c) arduino system, (d) sensitive scale, (e) ultrasound nano equipment, (f) indicator of thermal conductivity, (g) images depicting the process of measuring viscosity |
The nanofluid test device propels nanofluid and pure water to a flat solar collector from their tanks, while also detecting water and nanofluid velocities. Two iron bases held the fluid tank, the flat solar collector for the water and nanofluid systems, two flow meters, and digital temperature scales to create the test system. The main parts are listed in Table 1.
| Table 1 Main parts of the test system |
1.2 Instruments for Sensing Temperature and Associated Math for Calibration
The Arduino chip, the electrical circuit's most crucial component, recorded fluid temperatures at the entrances and exits of primary and secondary flat solar collector, as well as radiation intensity flow rate.A memory card retained Arduino data permanently. The study's variables fit the Arduino UNO's 12 connection points. Each system (pure water and nanofluid) is pre-programmed to record seven data points per hour: entrance temperature, exit temperature, absorption plate temperature, glass cover temperature, ambient temperature, flow rate amount, and radiation intensity. Fig. 2(c) shows data from four digital and four analog heat sensors coupled to Arduino systems (K and NTC).
A mercury thermometer and a Celsius-graded mercury thermometer are used to calibrate thermocouple sensors (0-100). Fig. 3 shows the mercury thermometer and temperature sensors calibrated for a flat solar collector. They record temperatures until the water in a glass container with frozen water at 0 ℃ reaches 100 ℃ and boil.
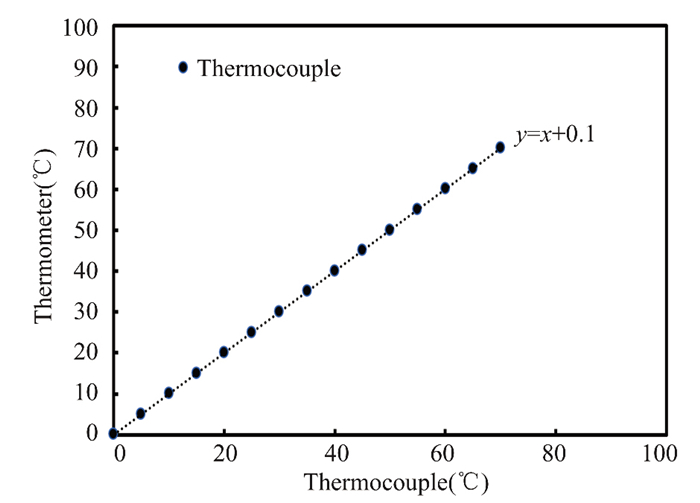
|
Fig.3 Temperature sensor calibration |
2 Preparation of Nanofluids
Nanofluid preparation begins with determining the volumetric ratio, or particle concentration in water, between the composition's water and nanoparticles. Titanium oxide (TiO2) nanoparticles, mixed and suspended in water to produce a nanofluid with specifications, were used in practical testing to evaluate the solar collector (A). According to Refs.[21-23], the following equation combines nanosolids with water:
| $m_{\mathrm{p}}=\frac{\emptyset \times \rho_{\mathrm{p}} \times\left(\frac{m_{\mathrm{f}}}{\rho_{\mathrm{f}}}\right)}{(1-\emptyset)}$ | (1) |
The equation itself is a calculation for the mass of the mixture (mp) based on the volume fraction of the solid phase (Ø), the density of the solid phase (ρp), the mass of the fluid phase (mf), the density of the fluid phase (ρf), and the complement of the volume fraction of the solid phase (1-Ø). Table 2 describes the characteristics of the TiO2.
| Table 2 Nanoparticles' thermal and physical characteristics |
Using Eq. (1), a sensitive scale was used to determine the number of nanoparticles needed to mix with water. Fig. 2(d) shows this scale device, while Fig. 2(e) shows the ultrasound nano equipment. There are two ways to combine solid nanoparticles with water after determining their quantity: We dissolved nanoparticles in water in two steps based on Refs. [24] and [25].
2.1 Second Procedure Entails the Use of an Ultrasound DeviceAn electric mixer combined nanoparticles and water for 40 min until it was completely homogeneous. An ultrasonic tool was used to evenly distribute nanoparticles in the mixture. This procedure involves many steps. The test equipment fed nanofluid into the basin and continuously mixed it with an electric mixer to suspend and disperse nanoparticles[26]. Monitoring the nanofluid till particle separation ensured water distribution. The volumetric stability constant of water-titanium oxide nanofluid peaks at 12 h (0.5%). The photos of the nanofluid samples being monitored are described in Fig. 4. The trials showed the stability of TiO2 in water.

|
Fig.4 Titanium dioxide at the nanoscale (0.5% TiO2-Water) being checked in action |
2.2 Physical Interpretations and Discussion
Nanofluid density, thermal conductivity, viscosity, and specific heat were measured experimentally.
2.2.1 DensityThe mathematical expression that relates to the density (ρ) is written as follows:
| $\rho=\frac{m}{v}$ | (2) |
where m is the mass, measured by a sensitive balance, and v is the water volume, measured by a graduated beaker.
2.2.2 Thermal conductivityHeat conduction is thermal conductivity. Conductivity Meters (TS-51) assessed water and nanofluid thermal conductivity with 0.01% accuracy, as shown in Fig. 2(f). The cylindrical component was inserted into the water sample, then the nanofluid sample was placed in the center of the beaker at the desired temperature. We reread the samples three times.
2.2.3 ViscosityThe Brookfield Digital Viscometer DV-Ⅱ+ Pro, as shown in Fig. 2(g), measures the viscosities of nanofluids and water using torque (shear stress) with a precision of 0.01%. Water and nanofluids were added, and viscosity was measured by rotating the device's bottom cylinder within the fluid's graded flask, as depicted in Fig. 2(g).
2.3 Solar Collector Configuration and Mathematical FormulationTwo flat solar collectors of identical size, one using distilled water and the other using a nanofluid with 0.5% titanium oxide, were installed on the roof of a Kirkuk residential house building for practical testing. Nine temperature sensors and a memory card reader gather the data. It stores discoveries in 16 GB of storage. These methods were used to study mass flow rate and radiation intensity on a flat solar collector in Kirkuk's climate in March and April. To install the solar collector, first, it is important to clean the compound's outside to remove any dust and dirt from the solar collector's glass. This will prevent the sun's energy from being weakened and skewing the data. Next, add water to the tank and distribute it via the pipe system using the relief valve. The grid-backed UPS and pipe water are connected through the system. A flow meter is used to measure water flow in the solar collector, as the mass flow was 0.02, 0.009, 0.009, 0.0045 kg/s. It is also important to check all sensor positions for accuracy before proceeding.
To ensure accurate readings, it is necessary to restart the Arduino before taking measurements. The measurements should be taken from 9∶00 a.m. to 17∶00 p.m., using the Arduino device to measure and record sun radiation for each hour of the test. Additionally, wind and ocean temperatures should be measured with meteorology. Record the mass flow for both collectors, which varies from 0.0045 to 0.02 kg/s during the hourly processes. Thermocouples, mass flux, and solar radiation intensity meters were used to show the error percentage. The error rate was less than 1% for temperatures, 2% for mass flow, and 0.5% for solar radiation.
The heat extraction coefficient (FR) is calculated as follows:
| $F_{\mathrm{R}}=\frac{\dot{m} c_{\mathrm{p}} F}{A_{\mathrm{c}} U_{\mathrm{L}}}\left(1-\mathrm{e}^{\frac{-A_{\mathrm{c}} U_{\mathrm{L}} F}{\dot{m} c_{\mathrm{pf}}}}\right)$ | (3) |
This equation involves variables such as mass flow rate (mc), specific heat capacity (cp), cross-sectional area (Ac), overall heat transfer coefficient (UL), and a coefficient (F). The useful heat gain (Qu) is estimated from Eq. (4).
| $Q_{\mathrm{u}}=A_{\mathrm{c}} F_{\mathrm{R}}\left[\tau \alpha I_{\mathrm{T}}-U_{\mathrm{L}}\left(T_{\mathrm{PM}}-T_{\mathrm{a}}\right)\right]$ | (4) |
The equation considers variables such as correction factor (τ), heat transfer coefficient (α), incident thermal radiation (IT), overall heat transfer coefficient (UL), mean temperature (TPM), and ambient temperature (Ta). The thermal efficiency (ηc) is evaluated from Eq.(5).
| $\eta_{\mathrm{c}}=\frac{Q_{\mathrm{u}}}{A_{\mathrm{c}} I_{\mathrm{T}}}$ | (5) |
To calculate entropy generated by thermos, the Eq. (6) below is used:
| $\dot{S}_{\text {gen, th }}=\dot{m} C_p \ln \left(\frac{T_{\mathrm{o}}}{T_{\mathrm{i}}}\right)-\left(\frac{\dot{Q}_{\mathrm{s}}}{T_{\mathrm{s}}}\right)+\left(\frac{\dot{Q}_{\mathrm{o}}}{T_{\mathrm{a}}}\right)$ | (6) |
In Eq.(6), the first term,
| $\eta_{\mathrm{ex}}=1-\frac{T_{\mathrm{a}} \dot{S}_{\mathrm{gen}}}{\left[1-\left(\frac{T_{\mathrm{a}}}{T_{\mathrm{s}}}\right)\right] \dot{Q}_{\mathrm{s}}}$ | (7) |
Thermally exergy destruction is calculated using Eq.(8) below:
| $\dot{E} x_{\text {dest, th }}=T_{\mathrm{a}} \dot{S}_{\text {gen, th }}$ | (8) |
The flat solar collector's heat transfer coefficient, heat energy obtained, heat extraction coefficient, total heat loss coefficient, entropy generated, exergy created, and practical and theoretical efficiency were perfected. The experiment utilized two case studies of solar collector. The first scenario involved using water to determine the efficiencey of the flat solar collector, which was found to be 0.0135% and 0.02%.
3 Thermophysical Characterization of Aqueous NanofluidTitania nanofluid's thermophysical properties were measured experimentally. Density, viscosity, and heat conductivity are thermodynamic properties. Fig. 5 shows that at a concentration of 0.5%, the thermal conductivity of the nanofluid is 10.2% higher than that of pure water at 30 ℃, and even higher than at 60 ℃. Nanofluid thermal conductivity of the nanofluid rises faster with temperature compared to pure water.
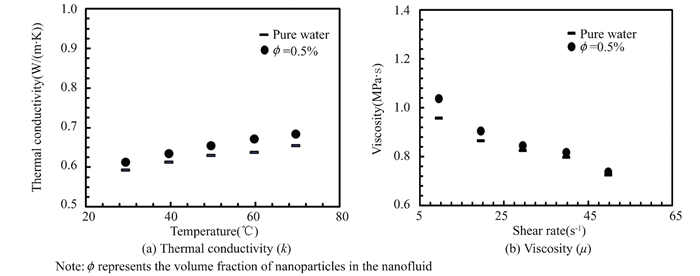
|
Fig.5 Thermal conductivity and viscosity |
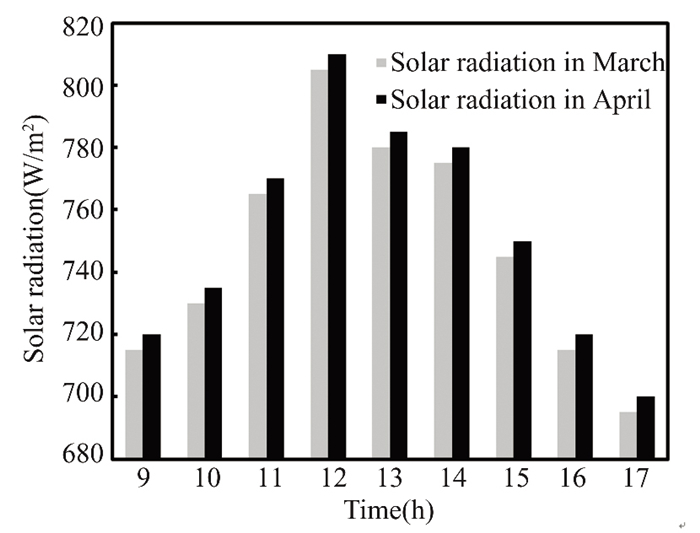
Between 9∶00 a.m. and 17∶00 p.m., time-lapse measurements of the nanofluid in the flat solar collector's temperature and mass flow rate were taken. This data was used to evaluate several critical parameters. Solar radiation peaks at 810 W/m2 at midday in April and 805 W/m2 in March before swiftly declining to its annual low towards the end of the year. The measurement in March was695 W/m2, while in April was 700 W/m2. Fig. 6 illustrates that the highest radiation intensity at noon in Kirkuk, will reach its peak in April 2022.
|
Fig.6 Average solar radiation intensity in Kirkuk, Iraq, in March and April, 2022 |
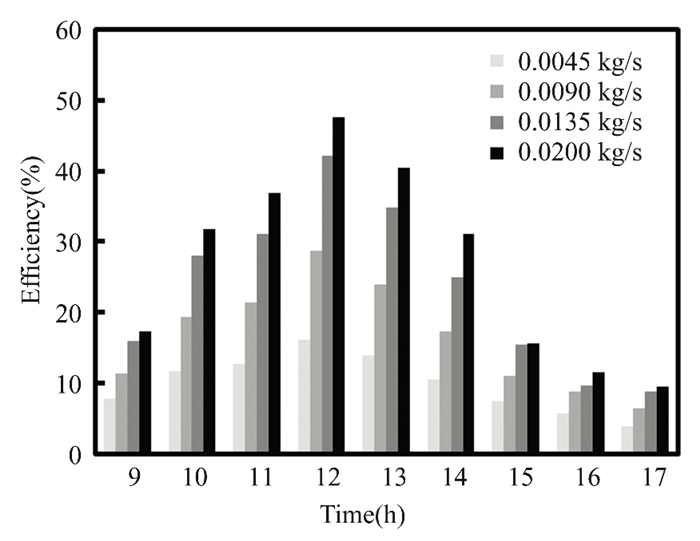
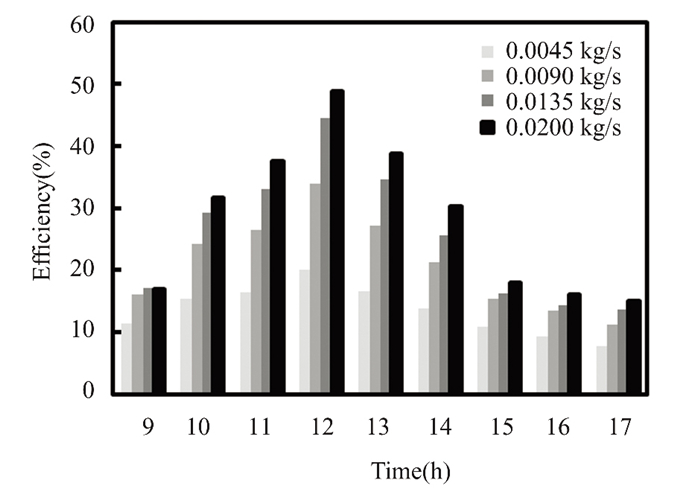
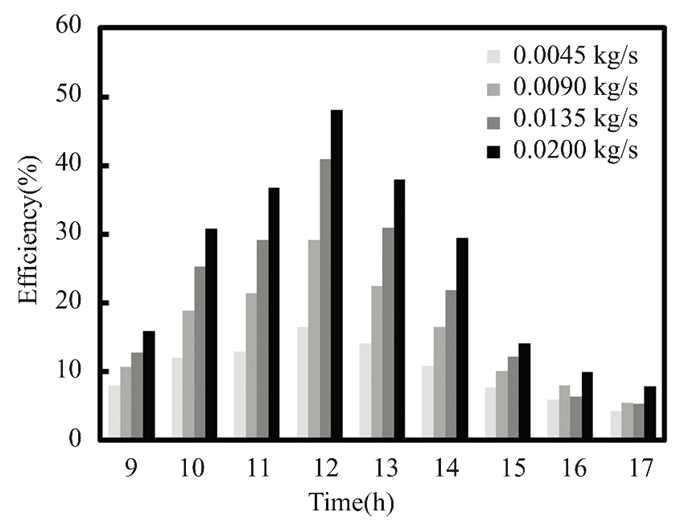
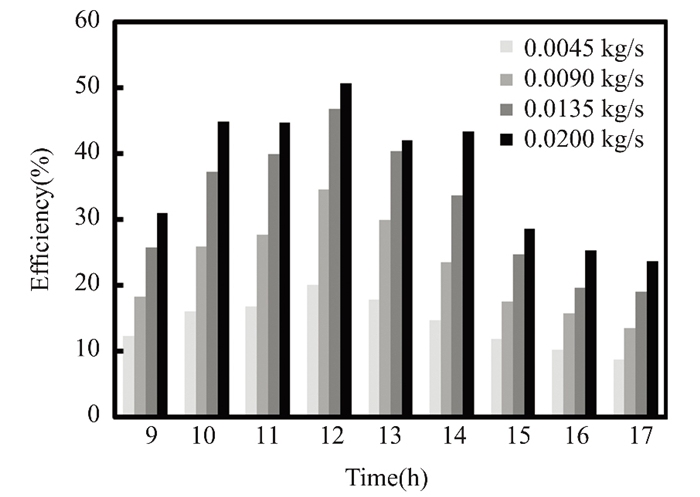
Due to solar radiation variation, water and titanium oxide collector efficiency climbed to 48% and 50% at noon in March, with a mass flow rate of 0.02 kg/s. As shown in Fig. 6, the maximum value of solar radiation is observed. Since energy is directly proportional to fluid mass flow rate, the solar collector's efficiency depends on solar radiation intensity. Titanium oxide nanofluids enhance collector performance due to their significant thermal expansion and improved natural convection, particularly at noon, the efficiency of nanofluid titanium oxide is 21% higher than that of pure water. At noon in April, when solar radiation peaked, with a mass flow rate of 0.2 kg/s, the instantaneous collector efficiency of water and titanium oxide reached 47.47% and 50.67%, respectively, as illustrated in Figs. 7-10. Nanofluids with 0.5% titanium oxide exhibit a 6.3% higher temperature than pure water at noon, when solar energy is at its greatest. The collector's efficiency decreases by 0.79% for pure water and 0.53% for titanium oxide at 5:00 p.m., when the mass flow rate remains at 0.2 kg/s.
|
Fig.7 Efficiency vs mass flow rate for nanofluids with pure water(The Kirkuk, Iraq, in March 2022) |
|
Fig.8 Efficiency vs mass flow rate for nanofluids with 0.5% TiO2(The Kirkuk, Iraq, in March 2022) |
|
Fig.9 Efficiency vs mass flow rate for nanofluids with pure water (The Kirkuk, Iraq, in April 2022) |
|
Fig.10 Efficiency vs mass flow rate for nanofluids with 0.5% TiO2(The Kirkuk, Iraq, in April 2022) |
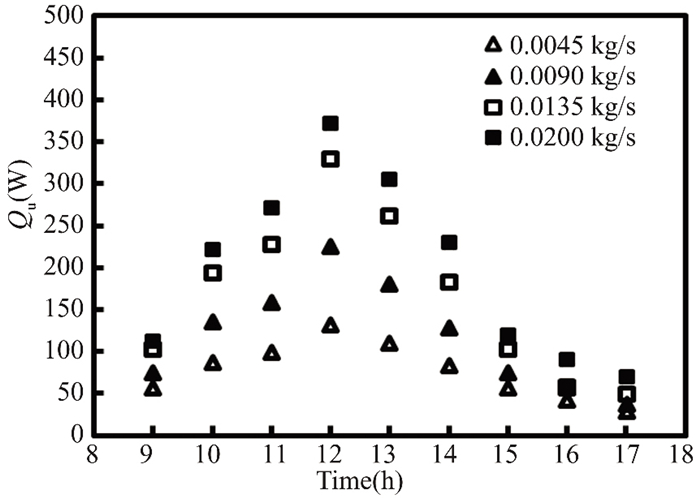
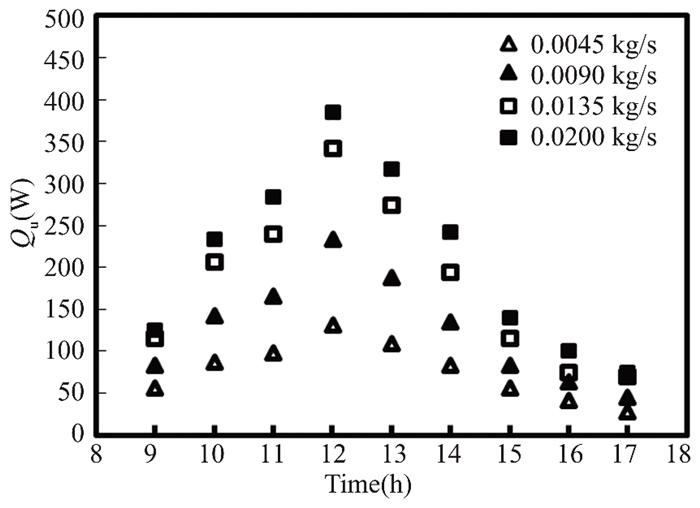
Figs. 11 and 12 show that the amount of heat energy gained practically by pure water from the solar collector rises with sunrise at various mass flow rates ranging from 0.0045 - 0.020 kg/s until it reaches its maximum value at noon due to the increase in radiation falling on the solar collector, and then it gradually decreases for two months (March and April). The compound time in April is greater than that in March. The highest usable energy is recorded in the forgotten month at a mass flow rate of 0.02 kg/s due to sun radiation variance.This is because solar energy density and fluid mass flow rate are related to useful energy.
|
Fig.11 The energy gained from pure water in Kirkuk, Iraq, in March 2022 compared to daylight hours |
|
Fig.12 The energy gained from pure water in Kirkuk, Iraq, in April 2022 compared to daylight hours |
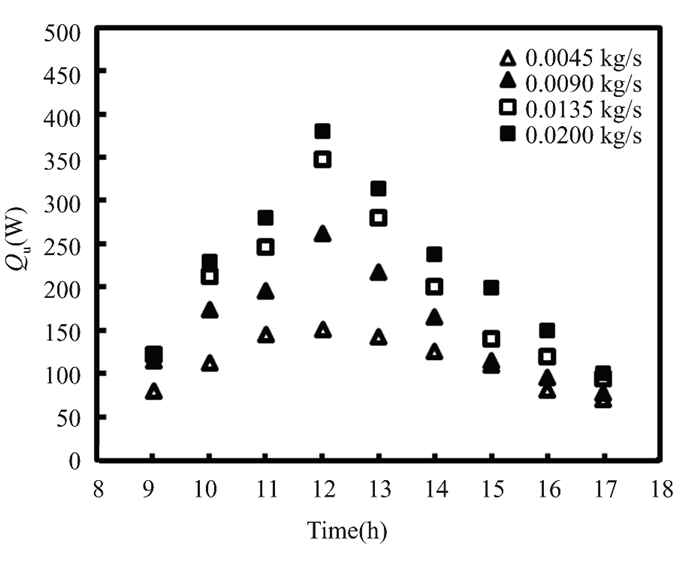
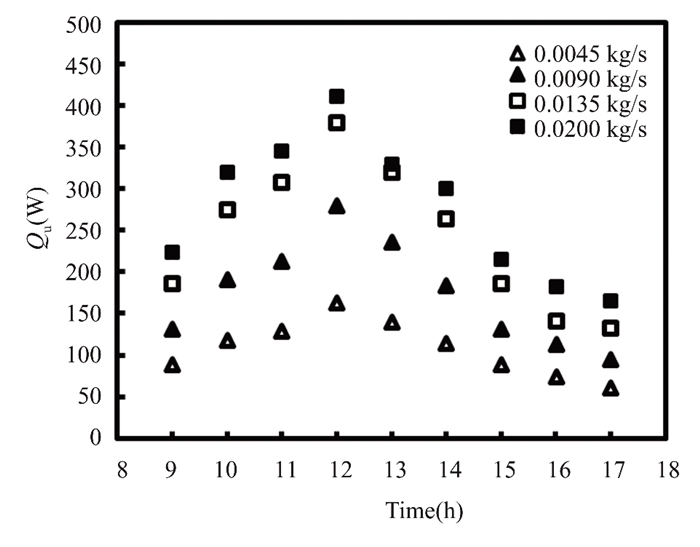
April's high air temperature reduced losses, increasing useful energy by 3.2% compared to March at the same mass flow rate. Figs. 13 and 14 illustrate how much thermal energy a titanium oxide nanofluid at 0.5% of the solar collector gains at mass flow rates from 0.0045 kg/s to 0.020 kg/s. After rising consistently throughout the morning, the radiation hitting the solar collector peaks at midday and diminishes over the next two months (March and April). In April, radiation intensity fell on the complex was higher than in March, thus forgetfulness peaked at about 410 W at noon. Due to sun radiation variance, we recorded the highest figure in the forgetful month at a mass flow rate of 0.02 kg/s. This is because solar energy density and fluid mass flow rate are related to useful energy. Nanofluid titanium oxide increases useable energy by 0.5% in March and 0.6% in April compared to water.
|
Fig.13 Gain in energy produced by employing 0.5% titanium oxide in March 2022 in Kirkuk, Iraq |
|
Fig.14 Gain in energy produced by employing 0.5% titanium oxide in April 2022 in Kirkuk, Iraq |
3.1 Thermal Entropy Generation
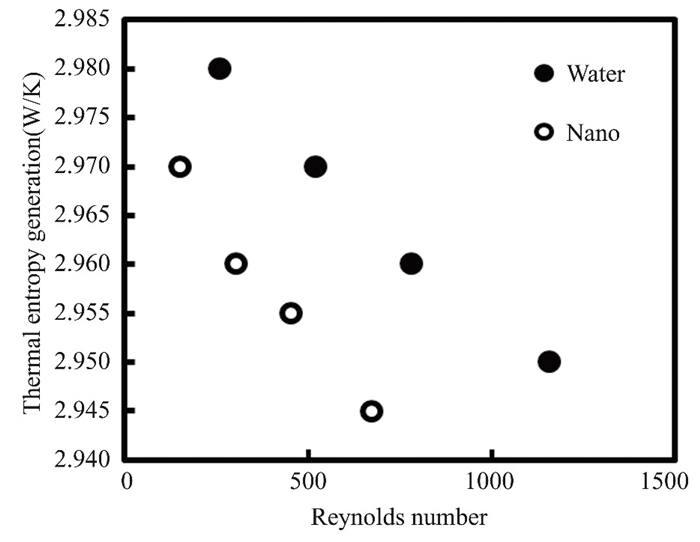
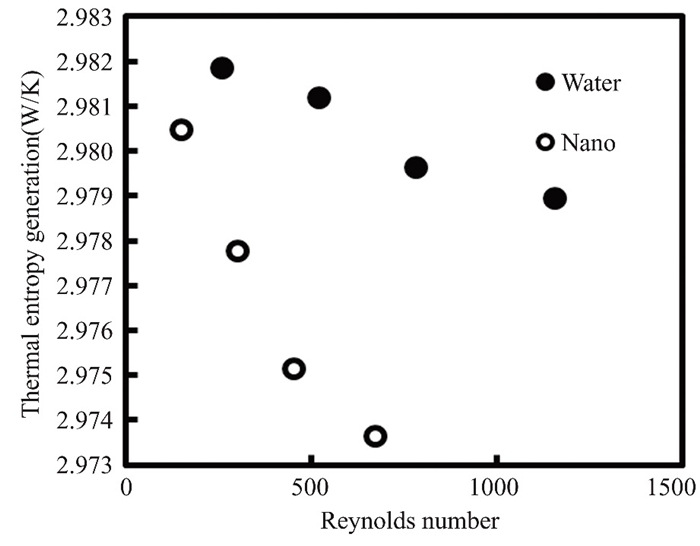
Thermal entropy is produced by thermodynamic processes. It is caused by heat transmission, fluid friction, and mixing. Thermal entropy, which is produced by all physical processes, severely restricts energy conversion. Thermal entropy generation is used by engineers and scientists to optimize power plants, refrigeration cycles, and heat exchangers. Reducing thermal entropy generation boosts efficiency while lowering environmental impact. The thermos generated entropy of the current method quantifies the performance degradation of the flat solar collector. The graphs depict the relationship between thermogenerated entropy and Reynolds number over the course of two months, as illustrated in Figs. 15 and 16. When the Reynolds numbers of pure water and titanium oxide nanofluid increase by 0.5%, thermal entropy falls. Pure water and titanium oxide had the highest levels of thermally generated entropy at 9∶00 a.m. in March and April, with values of 2.98 and 2.97 W/K, respectively. According to these findings, water has the highest value of produced entropy. Because the physical properties of nanofluids vary with volumetric concentration, the entropy value rises and reaches a minimum at midday, and the Reynolds number has a specific value for nanofluids. When solar radiation falls, thermal entropy rises. Collector efficiency and entropy generation aid in the evaluation of nanofluid-based FPCs.
|
Fig.15 The thermal entropy of both pure water and nanofluidrose by 0.5% in March 2022 in Kirkuk, Iraq |
|
Fig.16 The thermal entropy of both pure water and nanofluidrose by 0.5% in April 2022 in Kirkuk, Iraq |
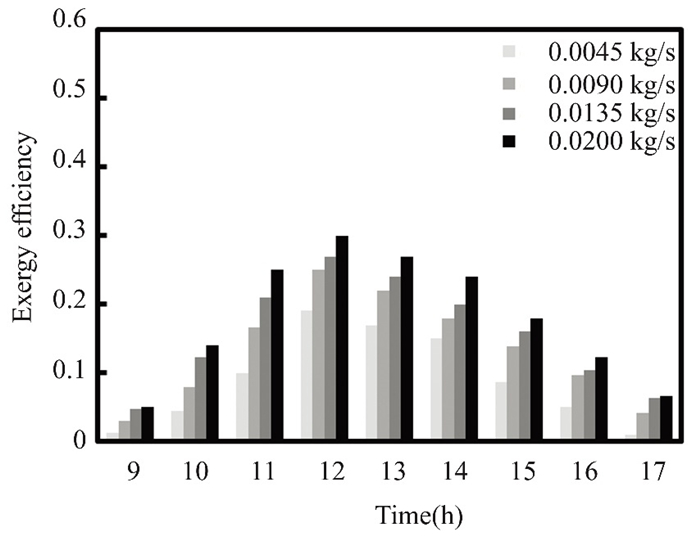
Flat solar collectors' exergy efficiency increases with sunlight in March, until reaches 0.0045 kg/s, as described in Figs. 17 and 18. The flat solar collector's efficiency drops as solar radiation intensity diminishes after midday in March and April. Nanofluids improve the physical parameters of the fluid used throughout the test, increasing the exergy efficiency of the flat solar collector by 0.4%, 0.3%, 0.2%, and 0.08% for flow masses of 0.02, 0.0135, 0.09, and 0.0045 kg/s. Figs. 19 and 20 show how increasing solar radiation in a flat solar collector boosts exergy efficiency in April. The flat solar collector's mass flux, which is 0.0045 kg/s during midday, is one factor that directly affects its efficiency. After midday, as solar radiation intensity decreases, the mass flux also decreases. Water containing 0.5% titanium oxide nanoparticles, at the flow rates of 0.02, 0.0135, 0.009, and 0.0045 kg/s, boosts the exergy efficiency.
|
Fig.17 Exergy efficiency over time in March of 2022 in Kirkuk, Iraq, at varied flows of pure water |
|
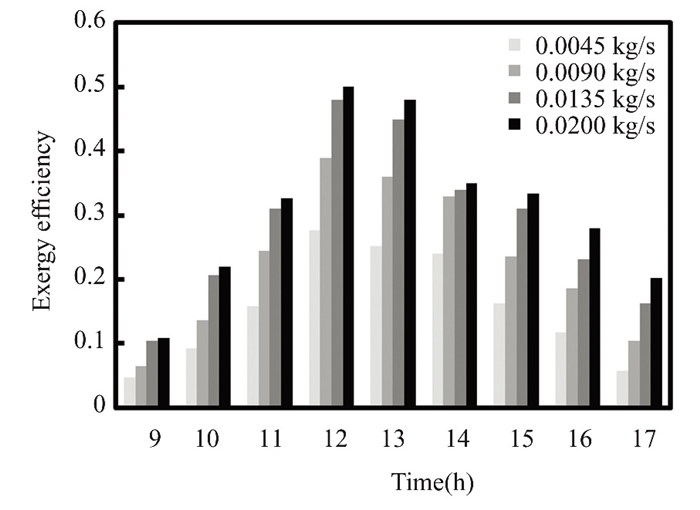
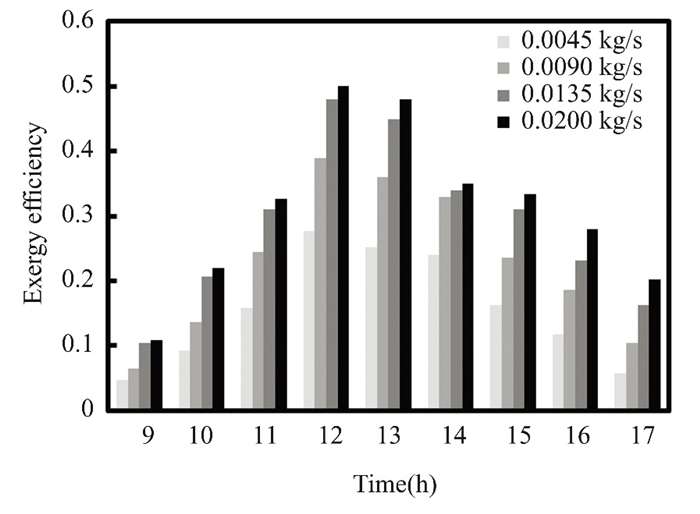
Fig.18 Exergy efficiency for nanofluid titanium oxide at 0.5% flow rate in March 2022 in Kirkuk, Iraq |
|
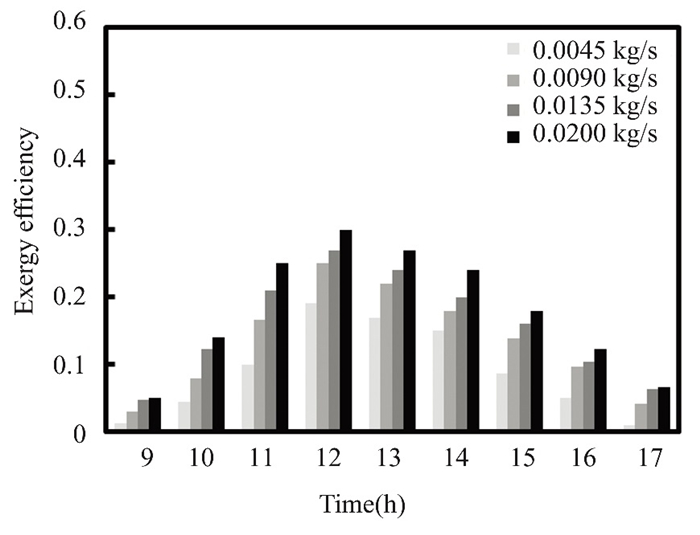
Fig.19 Exergy efficiency over time in April of 2022 in Kirkuk, Iraq, at varied flows of pure water |
|
Fig.20 Exergy efficiency for nanofluid titanium oxide at 0.5% flow rate in April 2022 in Kirkuk, Iraq |
3.2 Comparison of Exergy Efficiency with Thermal Efficiency
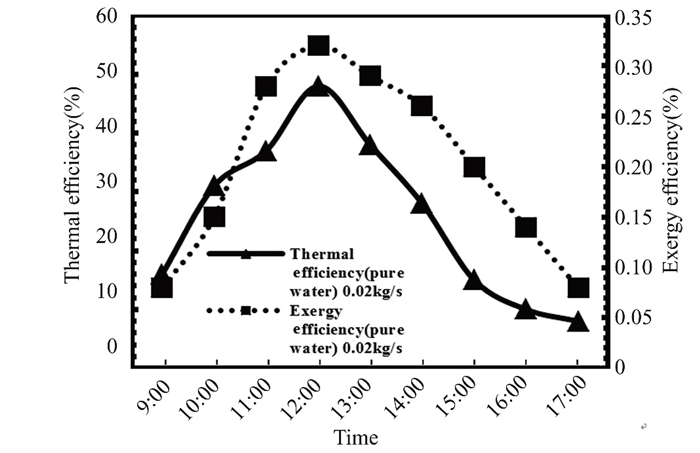
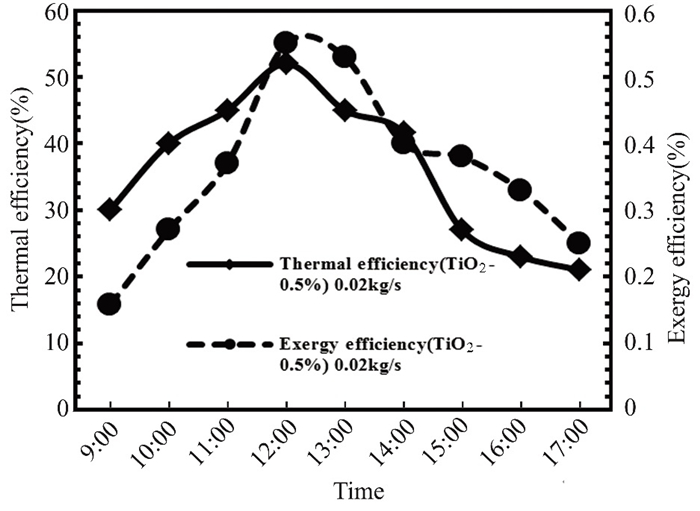
This study evaluates the efficiency and performance of flat solar collector. Additionally, it seeks to maximize thermo-exergy efficiency, which measures solar light-to-thermal energy conversion.Heat transfer, fluid dynamics, and system structure affect power utilization. A nanofluid improved the flat solar collector's physical characteristics and thermal losses, enhancing exergy efficiency. Researchers studied the solar collector's efficiency (practically and theoretically), entropy production, exergy creation, heat transfer coefficient, heat energy acquisition, heat extraction coefficient, total heat loss coefficient, and heat transfer coefficient. The study revealed that titanium oxide nanoparticles in flat solar collector water increased both thermal and exergy efficiency. Nanoparticles improved energy gain and reduced process losses, leading to an increase in exergy efficiency.Figs. 21 and 22 show Kirkuk's thermal and exergy efficiency at 0.02 kg/s for pure water and nanofluid titanium oxide. Solar radiation peaks between 9 a.m. and 12 p.m., while energy and thermal efficiency diminish between 12 p.m. and 5 p.m. Both pure water and nanofluid have better heat transmission, energy acquisition, and entry-exit temperature differential. As the day progresses and the temperature rises, thermal losses in the flat solar collector diminish, boosting the thermal and exergy efficiency of both clean water and nanofluid.
|
Fig.21 Thermal and exergy efficiency in March 2022 Kirkuk, Iraq, at 0.02 kg/s |
|
Fig.22 Thermal and exergy efficiency in April 2022 Kirkuk, Iraq, at 0.02 kg/s |
3.3 Exergy Destruction Changes Throughout Time
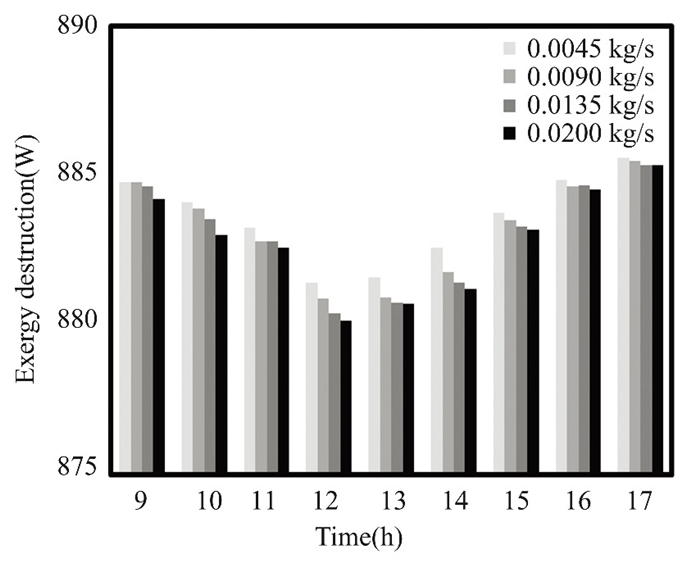
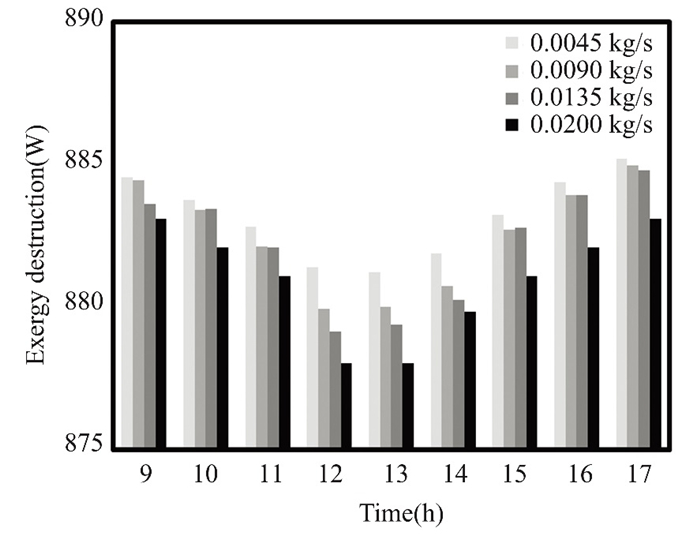
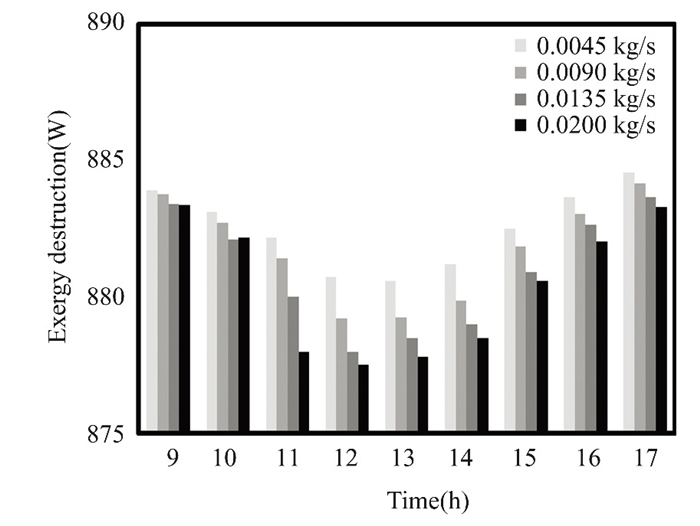
Operating conditions, input/output quality, and equipment age affect exergy destruction. Older equipment wastes energy. Pressure and temperature destroy system exergy. Fuel and waste quality affect exergy destruction efficiency. Monitoring exergy decay may show trends or outliers that affect system performance and longevity. The flat solar collector, which circulated clean water and nanofluids, experienced energy losses in March and April, as shown in Figs. 23-26. During these months, solar radiation is weakest, peaking around midday and declining after lunch. The collector operates with a flow rate of 0.02 kg/s. Thermal energy is strongest and energy loss is minimized during midday when solar radiation and ambient temperatures are at their peak. The addition of 0.5% titanium oxide particles in the nanofluid reduces energy loss.
|
Fig.23 Exergy destruction in March 2022, Kirkuk, Iraq, with changing pure water flow rates |
|
Fig.24 Exergy destruction in March with varied flow rates of 0.5% TiO2 nanofluid, Kirkuk, Iraq in 2022 |

|
Fig.25 Exergy destruction in April 2022, Kirkuk, Iraq, with changing pure water flow rates |
|
Fig.26 Exergy destruction in April & varied flow rates of 0.5% TiO2 nanofluid, Kirkuk, Iraq in 2022 |
4 Conclusions
The experiment showed that nanofluid titanium oxide can increase the efficiency of flat solar collectors in terms of heat energy gain and exergy efficiency. At noon, in March and April, with a mass flow rate of 0.02 kg/s, water and titanium oxide collector efficiency climbed to 48% and 50%, respectively, due to the relationship between solar energy density and fluid mass flow rate. The use of nanofluid titanium oxide increased usable energy by 0.5% in March and 0.6% in April compared to water. The thermal entropy generated by all physical processes restricts energy conversion, and reducing thermal entropy generation can boost efficiency while lowering environmental impact. The experiment showed that the flat solar collector's exergy efficiency increases with sunlight in March until it reaches 0.0045 kg/s, and the efficiency drops as solar radiation intensity diminishes after midday in March and April.
| [1] |
Yousefi T, Veisy F, Shojaeizadeh E, et al. An experimental investigation on the effect of MWCNT-H2O nanofluid on the efficiency of flat-plate solar collectors. Experimental Thermal and Fluid Science, 2012, 39: 207-212. DOI:10.1016/j.expthermflusci.2012.01.025 (  0) 0) |
| [2] |
Vijayakumaar S C, Lakshmi Shankar R, Babu K. Effect of CNT-H2O nanofluid on the performance of solar flat plate collector-an experimental investigation. Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies. Chennai, 2013, 197-199. DOI:10.1109/ICANMEET.2013.6609275 (  0) 0) |
| [3] |
Zamzamian A, KeyanpourRad M, KianiNeyestani M, et al. An experimental study on the effect of Cu-synthesized/EG nanofluid on the efficiency of flat-plate solar collectors. Renewable Energy, 2014, 71: 658-664. DOI:10.1016/j.renene.2014.06.003 (  0) 0) |
| [4] |
Nasrin R, Alim M A. Semi-empirical relation for forced convective analysis through a solar collector. Solar Energy, 2014, 105: 455-467. DOI:10.1016/j.solener.2014.03.035 (  0) 0) |
| [5] |
Moghadam A J, Farzane-Gord M, Sajadi M, et al. Effects of CuO/water nanofluid on the efficiency of a flat-plate solar collector. Experimental Thermal and Fluid Science, 2014, 58: 9-14. DOI:10.1016/j.expthermflusci.2014.06.014 (  0) 0) |
| [6] |
Michael J J, Iniyan S. Performance of copper oxide/water nanofluid in a flat plate solar water heater under natural and forced circulations. Energy Conversion and Management, 2015, 95: 160-169. DOI:10.1016/j.enconman.2015.02.017 (  0) 0) |
| [7] |
Said Z, Sabiha M A, Saidur R, et al. Performance enhancement of a Flat Plate Solar collector using Titanium dioxide nanofluid and Polyethylene Glycol dispersant. Journal of Cleaner Production, 2015, 92: 343-353. DOI:10.1016/j.jclepro.2015.01.007 (  0) 0) |
| [8] |
Salavati Meibodi S, Kianifar A, Niazmand H, et al. Performance enhancement of a flat plate solar collector using titanium dioxide nanofluid and Polyethylene Glycol dispersant. Internation Communcation in Heat and Mass Transfer, 2015, 65: 71-75. DOI:10.1016/j.icheatmasstransfer.2015.02.011 (  0) 0) |
| [9] |
Vakili M, Hosseinalipour S M, Delfani S, et al. Experimental investigation of graphene nanoplatelets nanofluid-based volumetric solar collector for domestic hot water systems. Solar Energy, 2016, 131: 119-130. DOI:10.1016/j.solener.2016.02.034 (  0) 0) |
| [10] |
Verma S K, Tiwari A K, Chauhan D S. Performance augmentation in flat plate solar collector using MgO/water nanofluid. Energy Conversion and Management, 2016, 124: 607-617. DOI:10.1016/j.enconman.2016.07.007 (  0) 0) |
| [11] |
Jouybari H J, Saedodin S, Zamzamian A, et al. Effects of porous material and nanoparticles on the thermal performance of a flat plate solar collector: An experimental study. Renewable Energy, 2017, 114: 1407-1418. DOI:10.1016/j.renene.2017.07.008 (  0) 0) |
| [12] |
Syam Sundar L, Singh M K, Punnaiah V, et al. Experimental investigation of Al2O3/water nanofluids on the effectiveness of solar flat-plate collectors with and without twisted tape inserts. Renewable Energy, 2018, 119: 820-833. DOI:10.1016/j.renene.2017.10.056 (  0) 0) |
| [13] |
Zarda F, Hussein A M, Danook S H, et al. Enhancement of thermal efficiency of nanofluid flows in a flat solar collector using CFD. Diagnastyka, 2022, 23(4): 1-9. DOI:10.29354/diag/156384 (  0) 0) |
| [14] |
Kiliç F, Menlik T, Sözen A. Effect of titanium dioxide/water nanofluid use on thermal performance of the flat plate solar collector. Solar Energy, 2018, 164: 101-108. DOI:10.1016/j.solener.2018.02.002 (  0) 0) |
| [15] |
Sharafeldin M A, Gróf G. Experimental investigation of flat plate solar collector using CeO2-water nanofluid. Energy Conversion and Management, 2018, 155: 32-41. DOI:10.1016/j.enconman.2017.10.070 (  0) 0) |
| [16] |
Tong Y, Lee H, Kang W, et al. Energy and exergy comparison of a flat-plate solar collector using water, Al2O3 nanofluid, and CuO nanofluid. Applied Thermal Engineering, 2019, 159: 113959. DOI:10.1016/j.applthermaleng.2019.113959 (  0) 0) |
| [17] |
Nirmala P N. Comparative studies on efficiency of single and double glassed solar water heater. Materials Today: Proceedings, 2021, 34(Part 2): 420-424. DOI:10.1016/j.matpr.2020.02.204 (  0) 0) |
| [18] |
Choudhary S, Sachdeva A, Kumar P. Influence of stable zinc oxide nanofluid on thermal characteristics of flat plate solar collector. Renewable Energy, 2020, 152: 1160-1170. DOI:10.1016/j.renene.2020.01.142 (  0) 0) |
| [19] |
Okonkwo E C, Wole-Osho I, Kavaz D, et al. Thermodynamic evaluation and optimization of a flat plate collector operating with alumina and iron mono and hybrid nanofluids. Sustainable Energy Technologies and Assessments, 2020, 37: 100636. DOI:10.1016/j.seta.2020.100636 (  0) 0) |
| [20] |
Alklaibi A M, Sundar L S, Sousa A C M. Experimental analysis of exergy efficiency and entropy generation of diamond/water nanofluids flow in a thermosyphon flat plate solar collector. International Communication in Heat and Mass Transfer, 2021, 120: 105057. DOI:10.1016/j.icheatmasstransfer.2020.105057 (  0) 0) |
| [21] |
Javadi F S, Sadeghiporu S, Saidur R, et al. The effects of nanofluid on thermophysical properties and heat transfer characteristics of a plate heat exchanger. International Communications in Heat and Mass Transfer, 2013, 44: 58-63. DOI:10.1016/j.icheatmasstransfer.2013.0 (  0) 0) |
| [22] |
Ramachandran K, Hussein A M, Kadirgama K, et al. Thermophysical properties measurement of nano cellulose in ethylene glycol/water. Applied Thermal Engineering, 2017, 123: 1158-1165. DOI:10.1016/j.applthermaleng.2017.05 (  0) 0) |
| [23] |
Ghorbani B, Akhavan-Behabadi M A, Ebrahimi S, et al. Experimental investigation of condensation heat transfer of R600a/POE/CuO nano-refrigerant in flattened tubes. International Communications in Heat and Mass Transfer, 2017, 88: 236-244. DOI:10.1016/j.icheatmasstransfer.2017.0 (  0) 0) |
| [24] |
Baby S, Johnson J. Numerical investigation on the heat transfer characteristics of alumina-water nanofluid in a double pipe heat exchanger. International Research Journal of Engineering and Technology (IRJET), 2018, 5(3): 3976-3983. (  0) 0) |
| [25] |
Hussein A M, Khaleell O S, Danook S H. Enhancement of double-pipe heat exchanger effectiveness by using water-CuO. NTU Journal of Engineering and Technololgy, 2022, 1(2): 18-22. DOI:10.56286/ntujet.v1i2.59 (  0) 0) |
| [26] |
Singh R N, Rajat P, Lav I, et al. Experimental studies of nanofluid TiO2/CuO in a heat exchanger (Double Pipe). Indian Journal of Science and Technology, 2016, 9(31): 1-6. DOI:10.17485/ijst/2016/v9i31/93623 (  0) 0) |
 2024, Vol. 31
2024, Vol. 31


